Preparation process for extracting human fibrinogens from waste for extracting cryoprecipitated blood coagulation factor VIII
A technology of human fibrinogen and coagulation factor, which is applied in the preparation methods of fibrinogen, coagulation/fibrinolytic factor, peptide, etc. The effect of reducing the incidence of adverse reactions, saving scarce plasma resources, and improving market competitiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
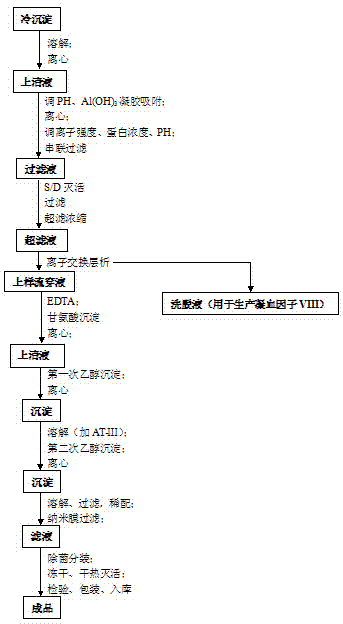
[0043] Embodiment 1: Taking 20,000 liters of plasma as an example, the specific preparation process is as follows:
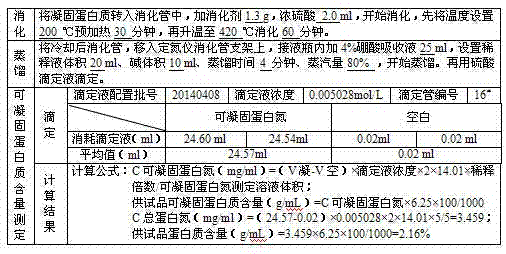
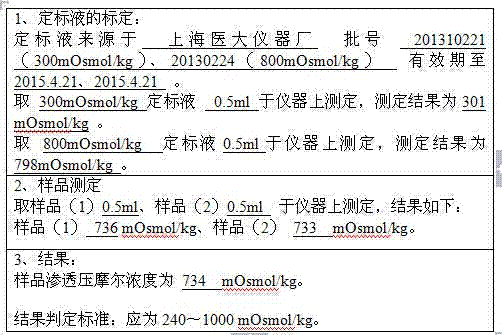
[0044] (1) During the quarantine period, after receiving the plasma of qualified individuals, wipe the surface of the plasma bag with 75% ethanol, rinse it with water for injection, merge it into a slurry tank, and melt it with circulating water below 30-35°C. The temperature of the plasma should not be higher than 4°C; after melting, centrifuge, control the liquid temperature at 0-4°C, and collect 136.9kg of cryoprecipitate;
[0045] (2) Add the cryoprecipitate obtained in step (1) into 3IU / ml heparin sodium solution, stir until the cryoprecipitate is completely dissolved, and control the temperature of the circulating water at 20-28°C; start centrifugation, collect the supernatant, Weighed 507.8kg;
[0046] (3) Adjust the pH of the supernatant obtained in step (2) to 6.6-7.2 with 0.5mol / L HCL; add 2% aluminum hydroxide gel, stir; start centrifugation, collect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com