Fibrin hydrogel stent loaded with human umbilical cord mesenchymal stem cells and application of fibrin hydrogel stent
A technology of stem cells and fibrin, applied in the field of fibrin hydrogel scaffolds, can solve the problems of high cost, long time, cumbersome preparation process, etc., achieve the effect of convenient material collection, promotion of re-epithelialization, and meeting treatment needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Primary isolation and culture of human umbilical cord mesenchymal stem cells
[0028] After the human umbilical cord is obtained from the operating table, it is immersed in PBS preservation solution containing 1% double antibody (penicillin) under aseptic conditions, the 50ml centrifuge tube is sealed and sterile, and it is transported to the laboratory in a 4°C ice box. Take it out in the ultra-clean workbench, wash it twice with 0.9% normal saline, remove the blood stasis at both ends, fully wash the periphery of the umbilical cord and the inner cavity of the umbilical vein with PBS buffer containing 1% double antibody, and remove the arteriovenous For blood vessels, take the umbilical cord matrix and cut into 4-5mm 3 Tissue pieces of different sizes were inoculated into T25 culture flasks containing 1ml DMEM / F12 (1:1) medium (containing 10% FBS), 5 pieces in each bottle, and a total of 10 bottles were placed in 37°C, 5% CO 2 , Culture in a saturated humi...
Embodiment 2
[0029] Example 2: Preparation of fibrin hydrogel scaffold loaded with human umbilical cord mesenchymal stem cells
[0030] A preparation method of a fibrin hydrogel scaffold loaded with human umbilical cord mesenchymal stem cells, specifically comprising the following steps:
[0031] (1) Human umbilical cord mesenchymal stem cells are subcultured after primary isolation and culture, and the subcultured P1-P6 generation human umbilical cord mesenchymal stem cells are cultured in culture flasks in a carbon dioxide incubator to make their density reach 70%-80%.
[0032] (2) Dissolving fibrinogen in sterile water for injection to prepare a fibrinogen solution (10 mg / ml).
[0033] (3) Dissolve thrombin in calcium ion solution (including 300mmol / L NaCl, 40mmol / L CaCl2) to prepare thrombin solution (25U / ml).
[0034] (4) Human umbilical cord mesenchymal stem cells were digested with 0.25% Trypsin-EDTA, and the resulting cells were resuspended with fibrinogen solution.
[0035] (5)...
Embodiment 3
[0036] Embodiment 3: Mouse full-thickness skin wound experiment
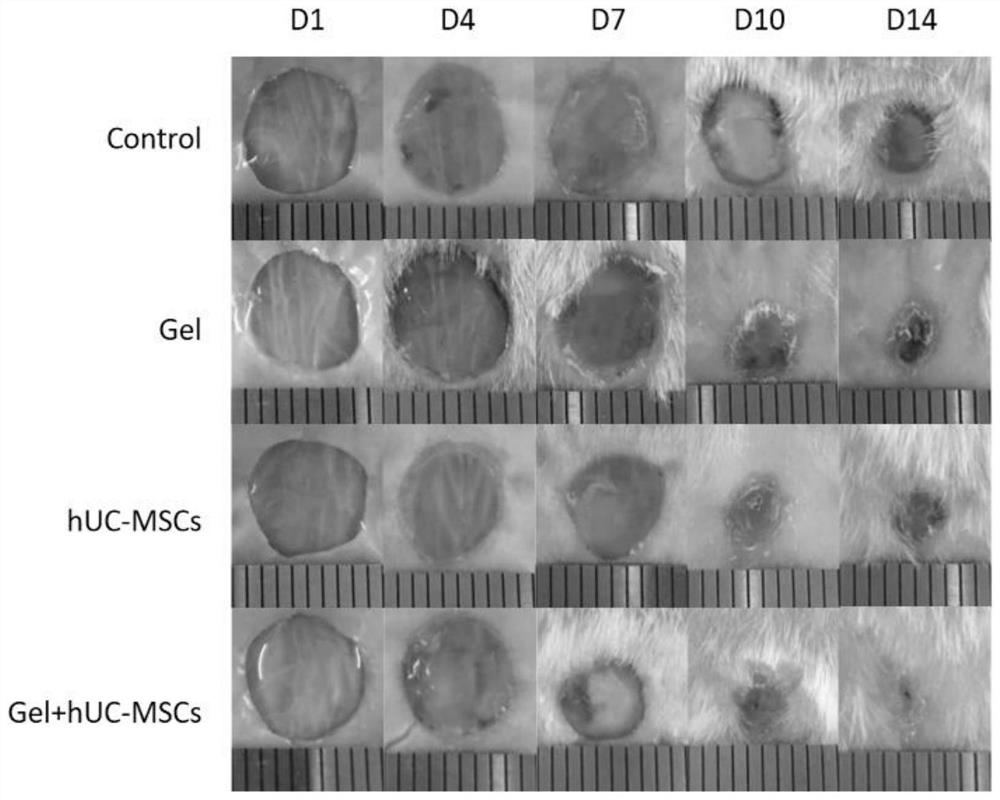
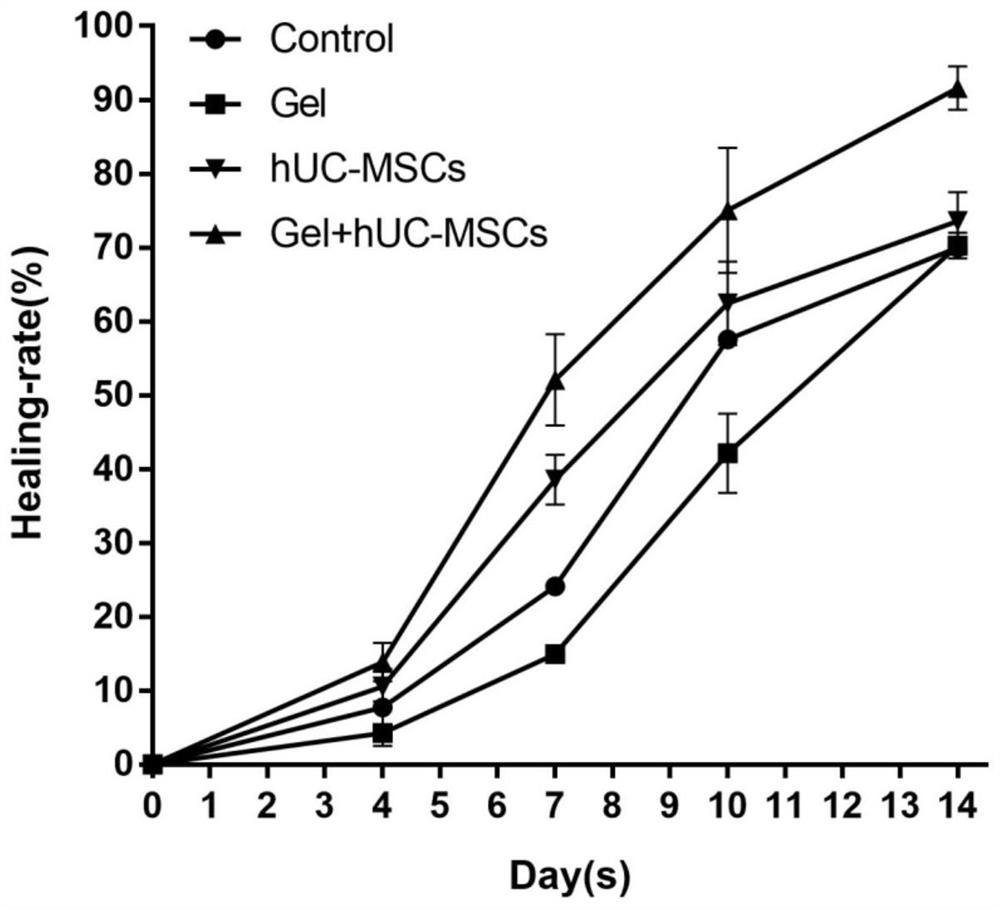
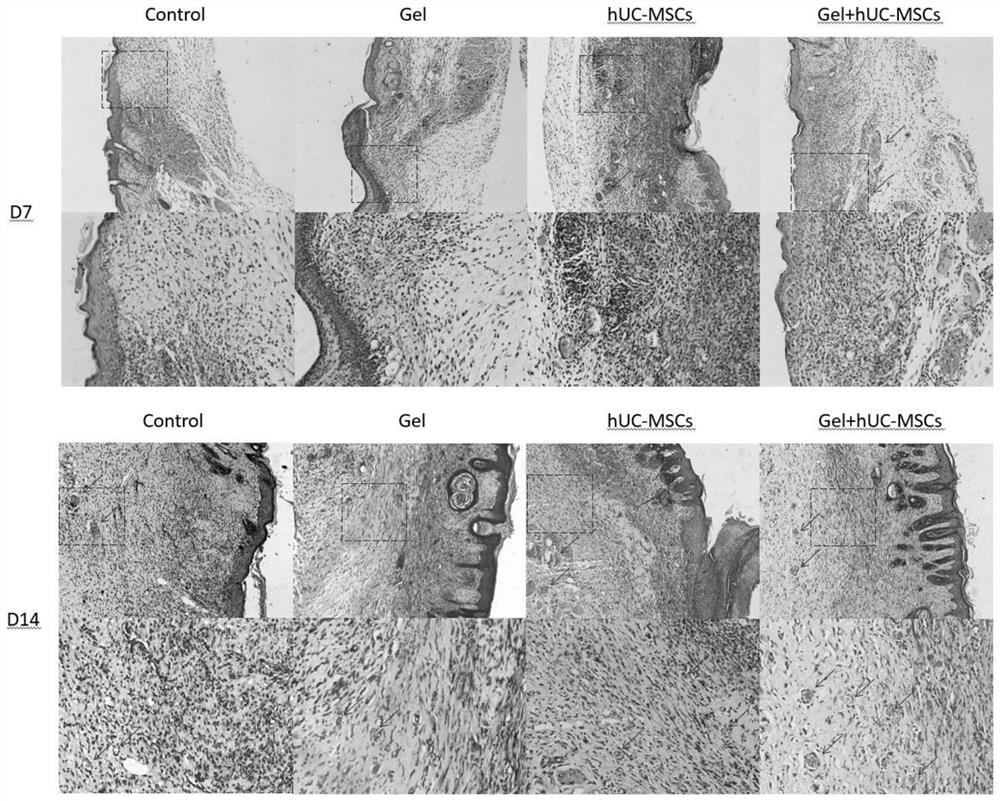
[0037] SPF grade BALB / C mice were used to construct a full-thickness skin wound model, and a fibrin hydrogel scaffold loaded with human umbilical cord mesenchymal stem cells was locally injected into the wound to evaluate its effect on skin wound healing.
[0038] (1) SPF grade BALB / C nude mice (8 weeks old, body weight 18-20g, female) were randomly divided into human umbilical cord mesenchymal stem cells + fibrin hydrogel group, human umbilical cord mesenchymal stem cell group, fibrin hydrogel group, Hydrogel group, saline group.
[0039] (2) Use an isoflurane gas anesthesia machine to induce anesthesia on mice with high concentration and high oxygen flow. After the anesthesia is satisfactory, fix them in the prone position on the operating table, maintain anesthesia at low concentration and low oxygen flow, and disinfect the back skin with Aner Iodine disinfectant three times.
[0040] (3) After laying a ste...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com