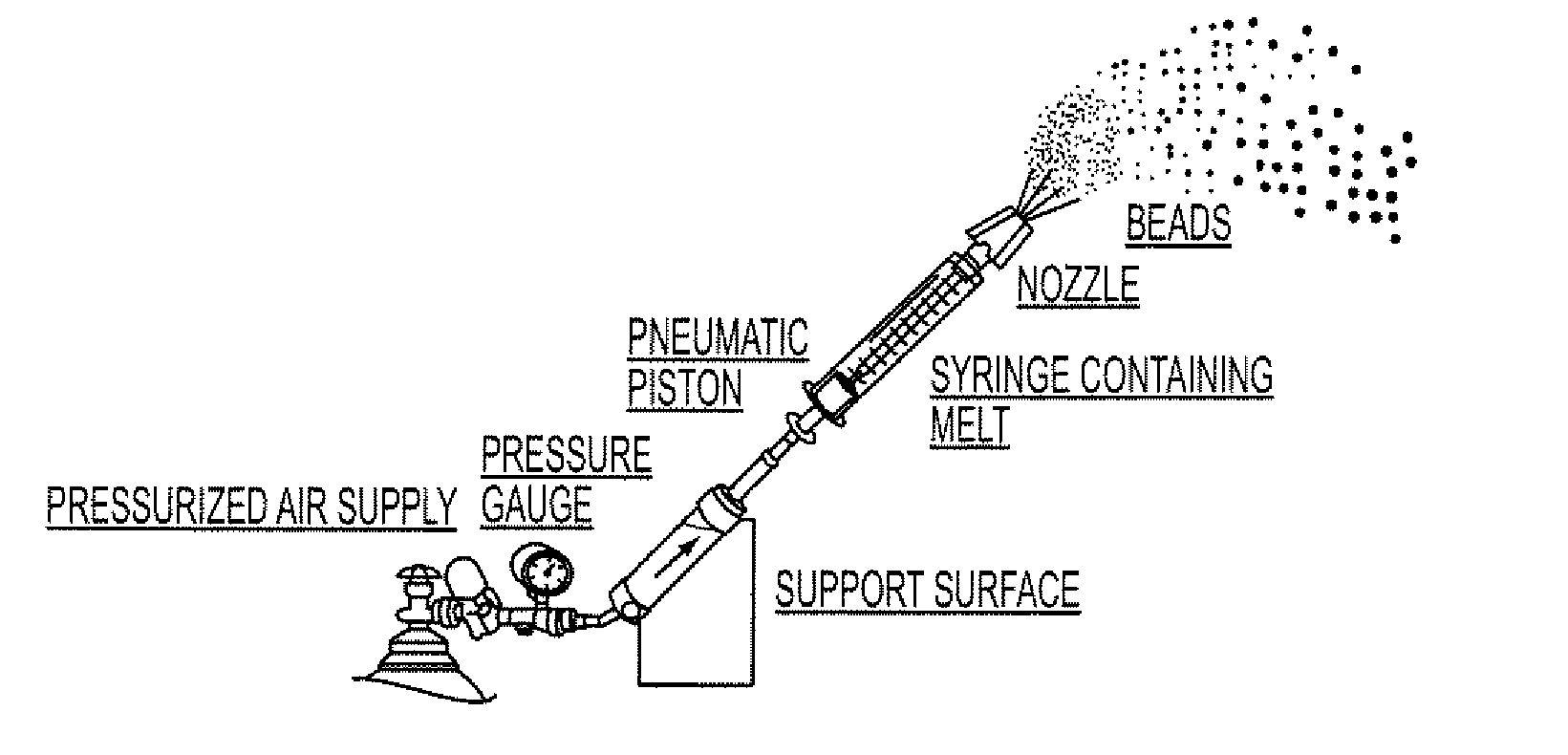
Tamper-resistant pharmaceutical compositions of opioids and other drugs
a technology of pharmaceutical compositions and opioids, applied in the field of pharmaceutical compositions, can solve problems such as abuse potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Drug Containing Multiparticulates
[0116]
TABLE 1CompositionsCompositionCompositionCompositionCompositionofofofofFormulationFormulationFormulationFormulationIngredientABCDOxycodone 5 g 5 g10 g 5 gBaseMyristic Acid——50 g30 gStearic Acid34 g34 g——Yellow10 g—10 g10 gBeeswaxCarnauba wax 5 g10 g20 g10 g
Procedure:
[0117]1. Fatty acid (myristic or stearic acid) was melted in an erlenmeyer flask in a silicone oil bath at 100° C. The mixture was stirred and kept under an argon blanket for this and all subsequent steps.
[0118]2. Oxycodone base was introduced into the molten fatty acid and the melt was stirred until the oxycodone base was completely dissolved and a clear liquid was formed.
[0119]3. Yellow beeswax was added and dissolved under constant stirring.
[0120]4. Carnauba wax was added and dissolved under constant stirring.
[0121]5. The resulting homogenous molten solution was poured onto aluminum foil and allowed to solidify at room temperature.
[0122]6. The bulk material obtaine...
example 2
Release of Drug from Crushed Multiparticulates
[0124]In vitro testing was conducted in order to assess the influence of crushing of the multiparticulates produced in Example 1 on the release in simulated stomach conditions. A currently marketed sustained release formulation of oxycodone, OxyContin®, was also subjected to crushing and dissolution for comparison purposes.
[0125]Multiparticulates (Formulations A, B, C or D, all 20-40 mesh in starting particle size) and OxyContin® tablets were crushed using a glass mortar and pestle. The resulting crushed material was placed in a dissolution vessel equipped with paddles (USP Apparatus II). 900 mL of 0.1N HCl pre-warmed to 37° C. was added to the vessels and stirred for 15 minutes. After 15 minutes the amount of oxycodone released was determined. The results are shown in Table 2.
TABLE 2Drug Release from Crushed Compositions% Relaeased in 15minutes in 0.1NSampleHCl (n = 3)Oxycontin ®95.6 + / − 2.7(40 mg Tablet)Formulation A31.6 + / − 2.6(multi...
example 3
Preparation of Oxycodone Containing Multiparticulates Using a Spinning Disc Atomization Process
[0127]Batch size: 1000 g
TABLE 3CompositionComponentQuantity(g) / BatchOxycodone base91Myristic acid545Beeswax182Carnauba Wax182Total1000.0
Procedure:
[0128]1. Myrisitc acid was melted at 85° C. in a silicone oil bath while constantly flowing argon above the surface of the solution.
[0129]2. Beeswax was added to the molten fatty acid and mixed until a clear, homogenous solution was obtained.
[0130]3. Carnauba wax was added to the molten solution and mixed until a clear, homogenous solution was obtained.
[0131]4. Oxycodone base was added to the molten solution and mixed until a clear, homogenous solution was obtained.
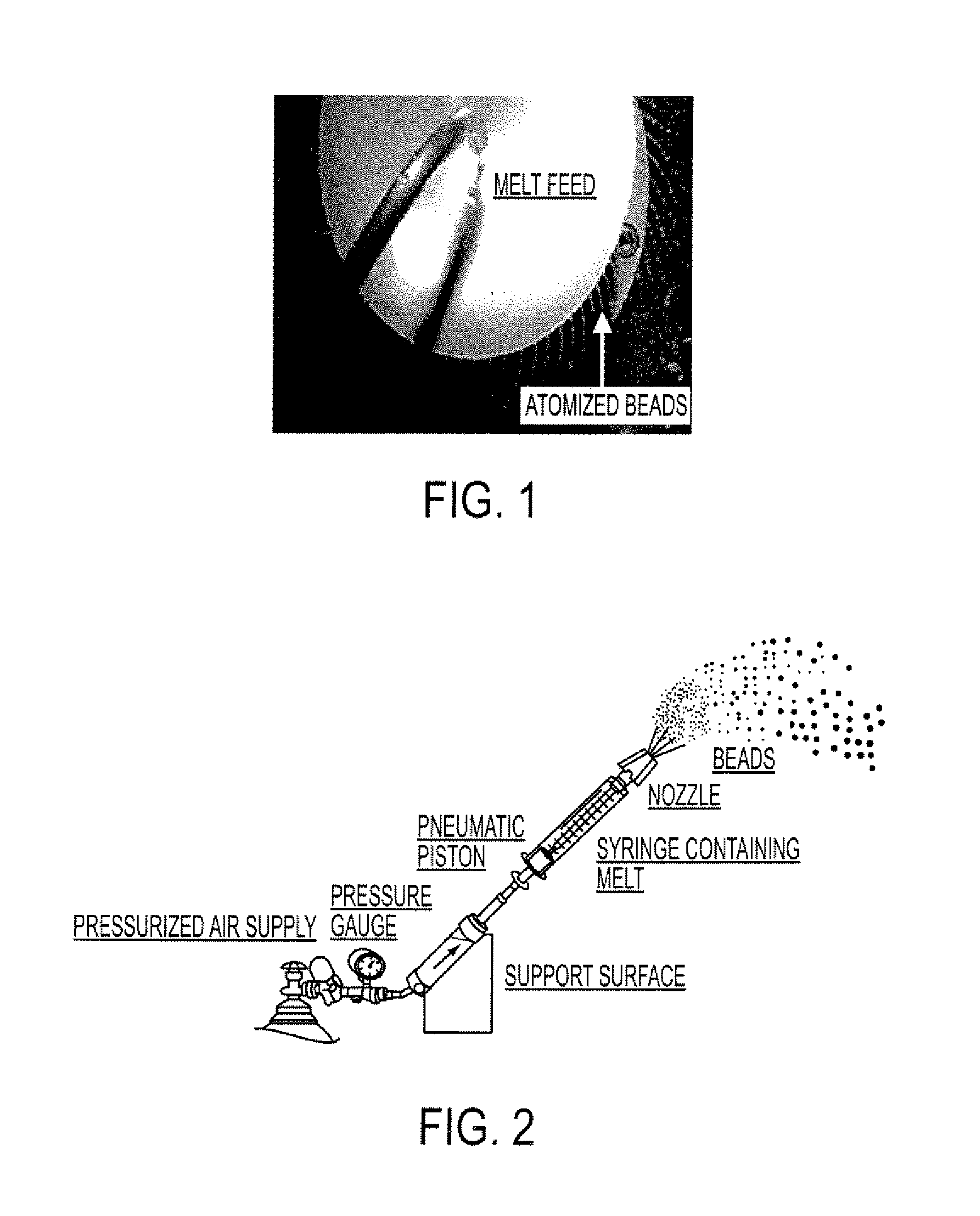
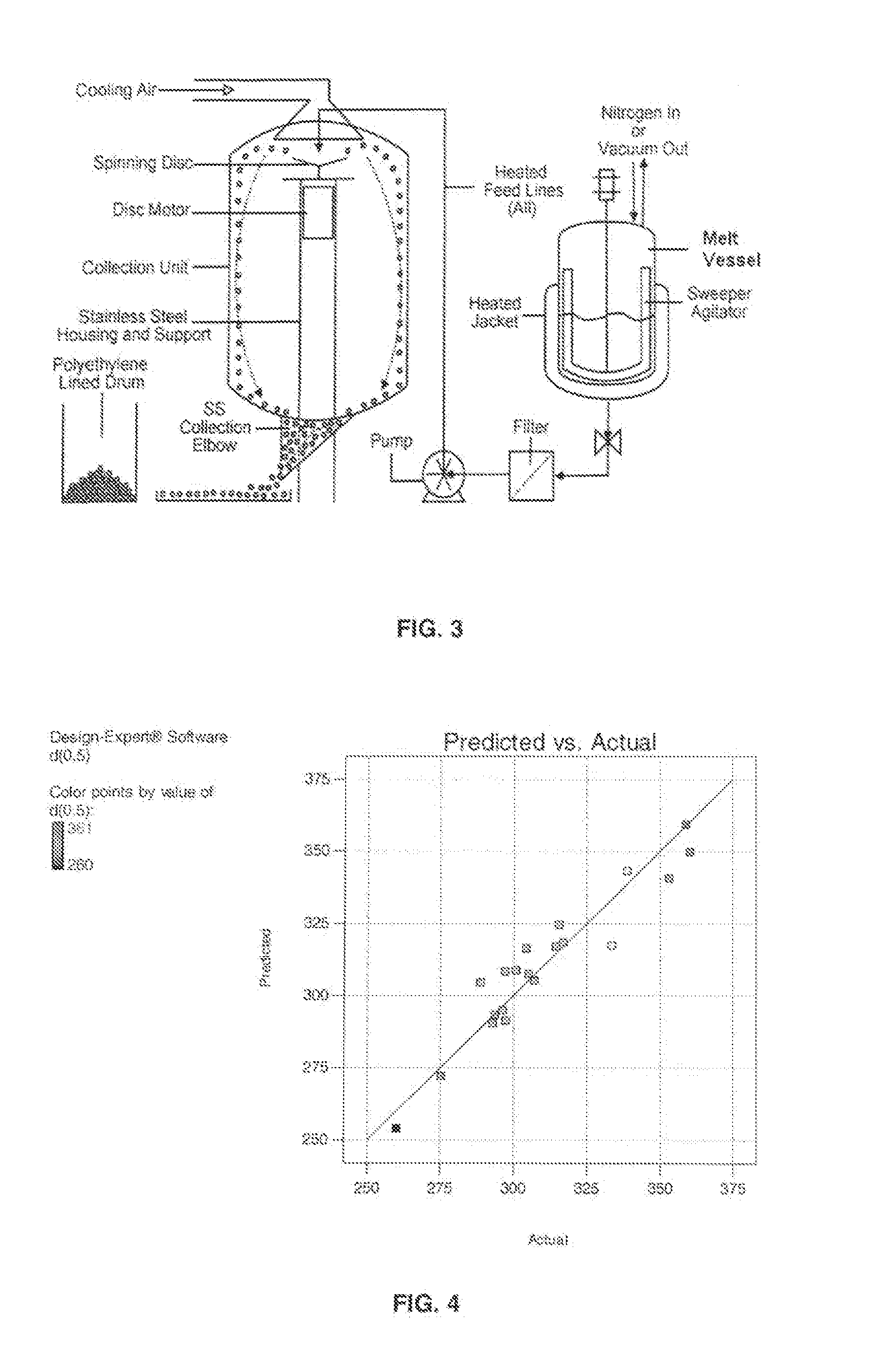
[0132]The resulting molten solution was transferred to a feed kettle and continuously metered onto a spinning disc atomizer (see FIG. 1) in order to form solid, spherical multiparticulates. These multiparticulates can be optionally spay coated with, for example, cellulose acetate pht...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com