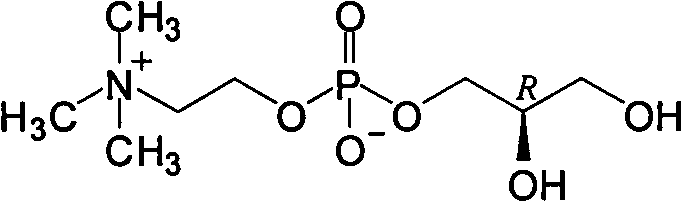
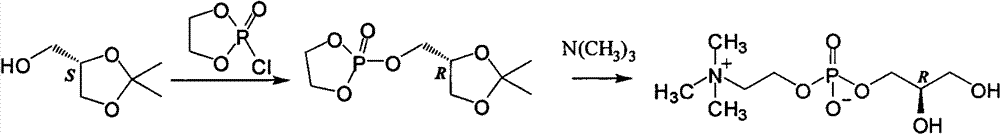
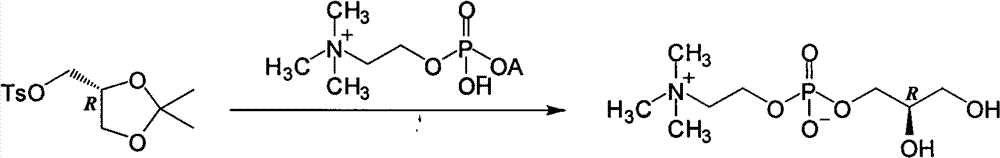
L-alpha-choline glycerophosphate synthesis method
A technology of glycerophosphocholine and a synthetic method, applied in the field of chiral synthetic chemistry and medicinal chemistry, can solve the problems of trivial details, unstable purification of glycerol, high cost of D-isopropylidene glycerol, etc., and achieves concise steps and high yield High, easily obtainable results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1, the preparation of phosphorylcholine tetramethylammonium salt
[0033] 100g (0.3mol) phosphorylcholine chloride calcium salt tetrahydrate was dissolved in 350mL water, heated to 50°C, 27g (0.3mol) oxalic acid was added thereto, stirred and reacted, and the calcium ion was completely precipitated, filtered with suction to obtain The filtrate was concentrated to dryness under reduced pressure under heating to obtain an oily substance, which was phosphorylcholine chloride. Add 300mL methanol to it, stir evenly, then add 54g (0.3mol) tetramethylammonium hydroxide pentahydrate, stir until completely dissolved, then remove the solvent under vacuum at 50°C to obtain 87.7g phosphorylcholine tetramethylammonium salt .
Embodiment 2
[0034] Embodiment two, the preparation of L-alpha-glycerophosphorylcholine crude product
[0035] Dissolve 87.7g (0.3mol) phosphorylcholine tetramethylammonium salt in 300mL water, add 33g (0.3mol) (R)-(-)-3-chloro-1,2-propanediol, heat to reflux overnight, cool, The water was distilled off under reduced pressure, 300 mL of ethanol was added to the residue, the slurry was beaten, the ammonium salt was removed by filtration, and the ethanol was distilled off the filtrate under reduced pressure to obtain about 70 g of crude L-α-glycerophosphorylcholine.
Embodiment 3
[0036] Embodiment three, the preparation of L-alpha-glycerophosphorylcholine crude product
[0037] Dissolve 87.7g (0.3mol) phosphorylcholine tetramethylammonium salt in 300mL methanol, add 33g (0.3mol) (R)-(-)-3-chloro-1,2-propanediol, heat to reflux overnight, cool , filtered to remove the ammonium salt, and the filtrate was evaporated to remove the methanol under reduced pressure to obtain about 71 g of the crude product of L-α-glycerophosphocholine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com