Humanized monoclonal antibody targeting to human CD19 antigen
A monoclonal antibody, humanized technology, applied in anti-animal/human immunoglobulin, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, anti-tumor drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1, design the humanized monoclonal antibody of anti-human CD19 antigen
[0045] (1) Amino acid sequences of the CDR regions of the mouse anti-human CD19 antigen monoclonal antibody strain FMC63 light and heavy chain variable regions such as SEQ ID NO.15, SEQ ID NO.16, SEQ ID NO.17 and SEQ ID NO.18 , shown in SEQ ID NO.19, SEQ ID NO.20. The sequence was analyzed and compared with the IMGT / BLAST database, and the human antibody sequence with the highest homology was selected as the transformation template.
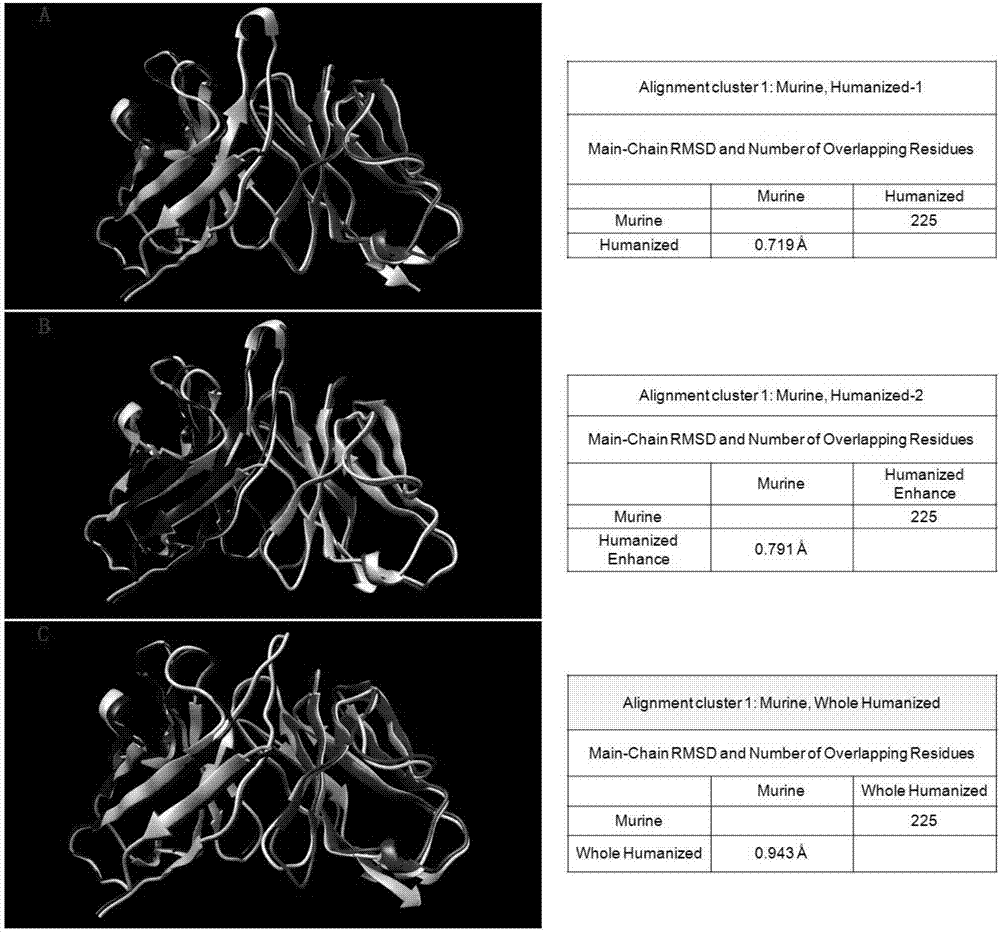
[0046] (2) After molecular docking simulation, the antibody amino acid residues most closely related to antigen-antibody docking are shown in Table 1. The framework sequences other than the key residues were humanized and modified with reference to the human antibody framework sequence, and then The humanized antibody is randomly mutated, and then the affinity is evaluated and predicted by the method of antigen-antibody affinity screening and molecular doc...
Embodiment 2
[0050] Example 2. Preparation of lentivirus expressing a chimeric antigen receptor targeting human CD19 antigen (CD19)
[0051] (1) Prepare the gene sequence of the chimeric antigen receptor targeting human CD19 antigen
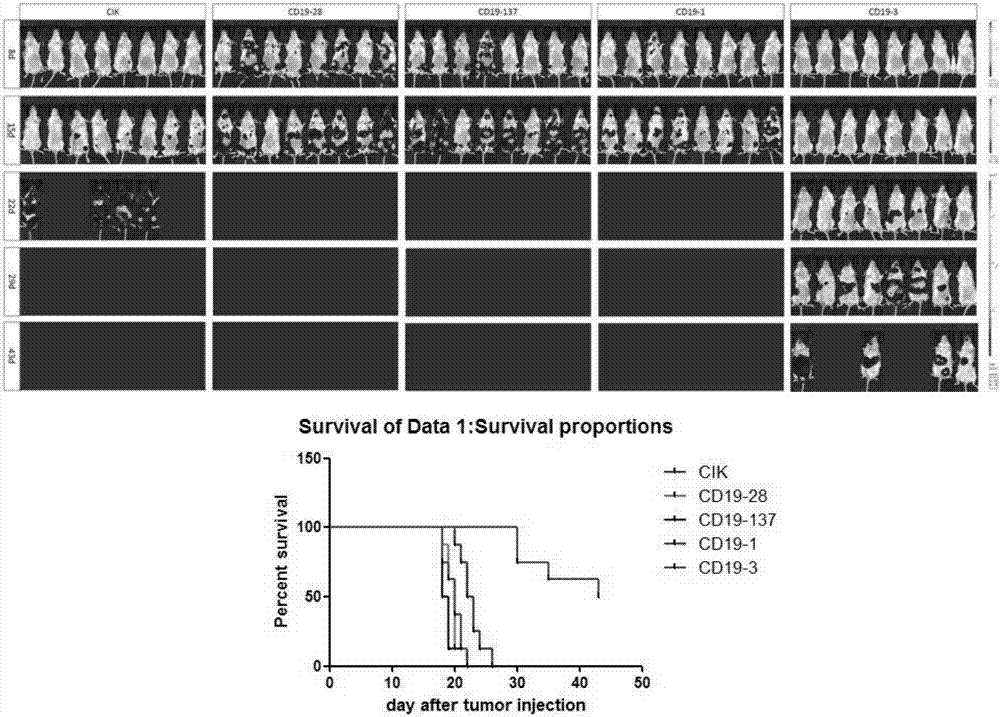
[0052] Synthesize chimeric antigen receptor sequences containing leader peptide (also known as signal peptide), anti-human CD19 antigen single-chain antibody ScFv, hFc hinge region, transmembrane region and intracellular signal segment in sequence, its structure is as follows image 3 shown. The nucleotide sequence of the leader peptide is shown in SEQ ID NO.5, and the amino acid sequence is shown in SEQ ID NO.6; humanized single-chain antibodies hCD19-1, hCD19-2, and hCD19-3 against human CD19 antigen The amino acid sequence is shown in SEQ ID NO.1, SEQ ID NO.2, SEQ ID NO.3, and the nucleotide sequence is shown in SEQ ID NO.21, SEQ ID NO.22, SEQ ID NO.23, as The amino acid sequence of the single-chain antibody CD19 derived from the control mouse anti-human C...
Embodiment 3
[0057] Example 3, Preparation of Chimeric Antigen Receptor Modified T Cells for CD19 Antigen
[0058] (1) Packaging of lentivirus
[0059] In this embodiment, the calcium phosphate method is used for packaging lentivirus, and the specific steps are as follows: 293T cells are cultivated to a better state with DMEM medium containing 10% FBS (w / v); 5 / cm 2 Density Pass up to 1 75cm 2 Incubate in a culture flask for 22 hours to ensure that the confluence of the cells is 70-80% during transfection; then replace the medium with preheated DMEM medium containing 2% FBS (w / v), and cultivate for 2 hours for later use; 680 μL ddH 2 O. 20 μg lentiviral vector (respectively mCD19, hCD19-1, hCD19-2, hCD19-3 lentiviral vector), 100 μL 2.5mM CaCl 2 Add it into a 15mL centrifuge tube, mix evenly, add 2×HBS dropwise to the mixture with a pipette after mixing, and at the same time, blow and mix with a 10mL pipette, let it stand at room temperature for 15min after mixing, and then transfer the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com