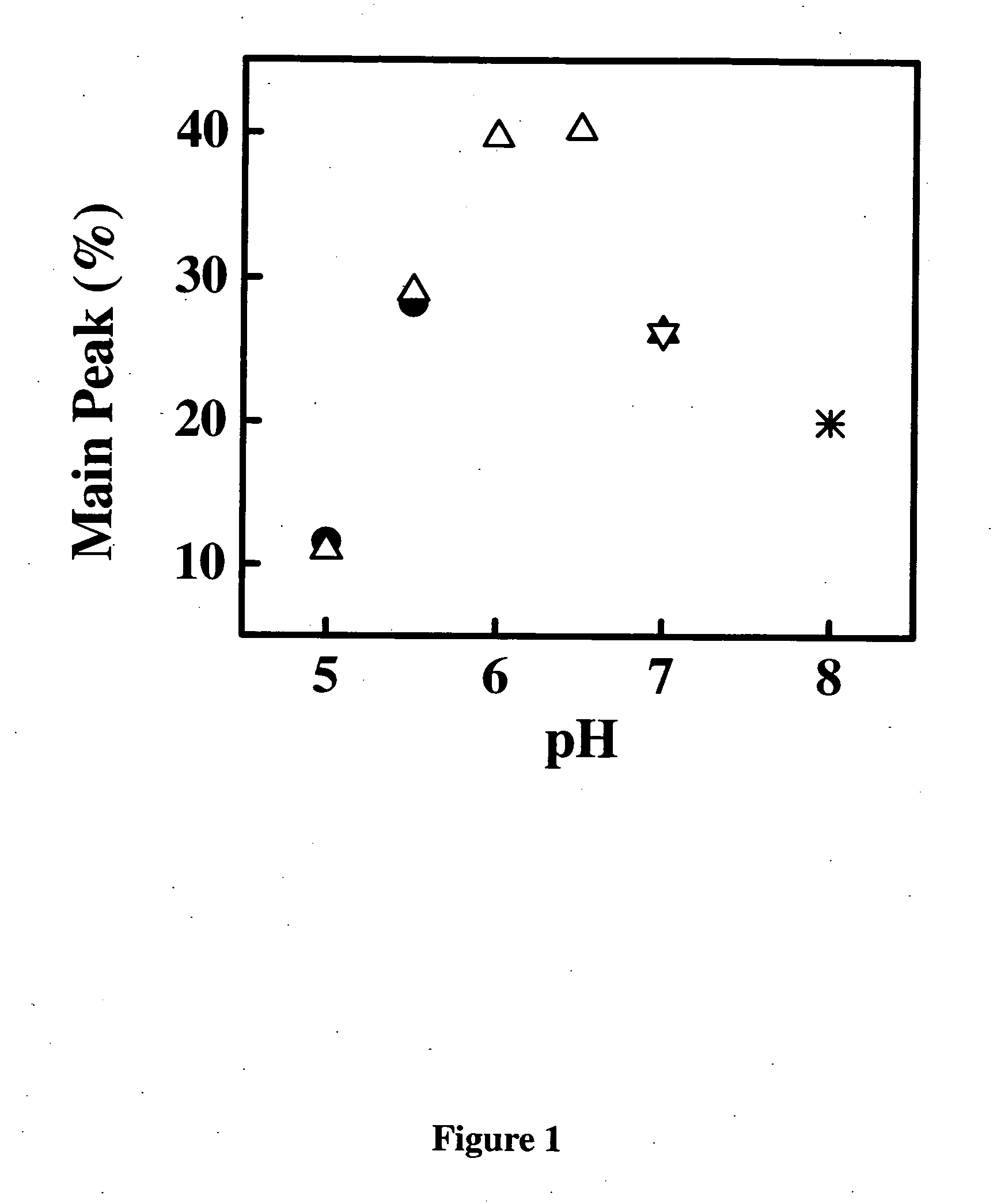
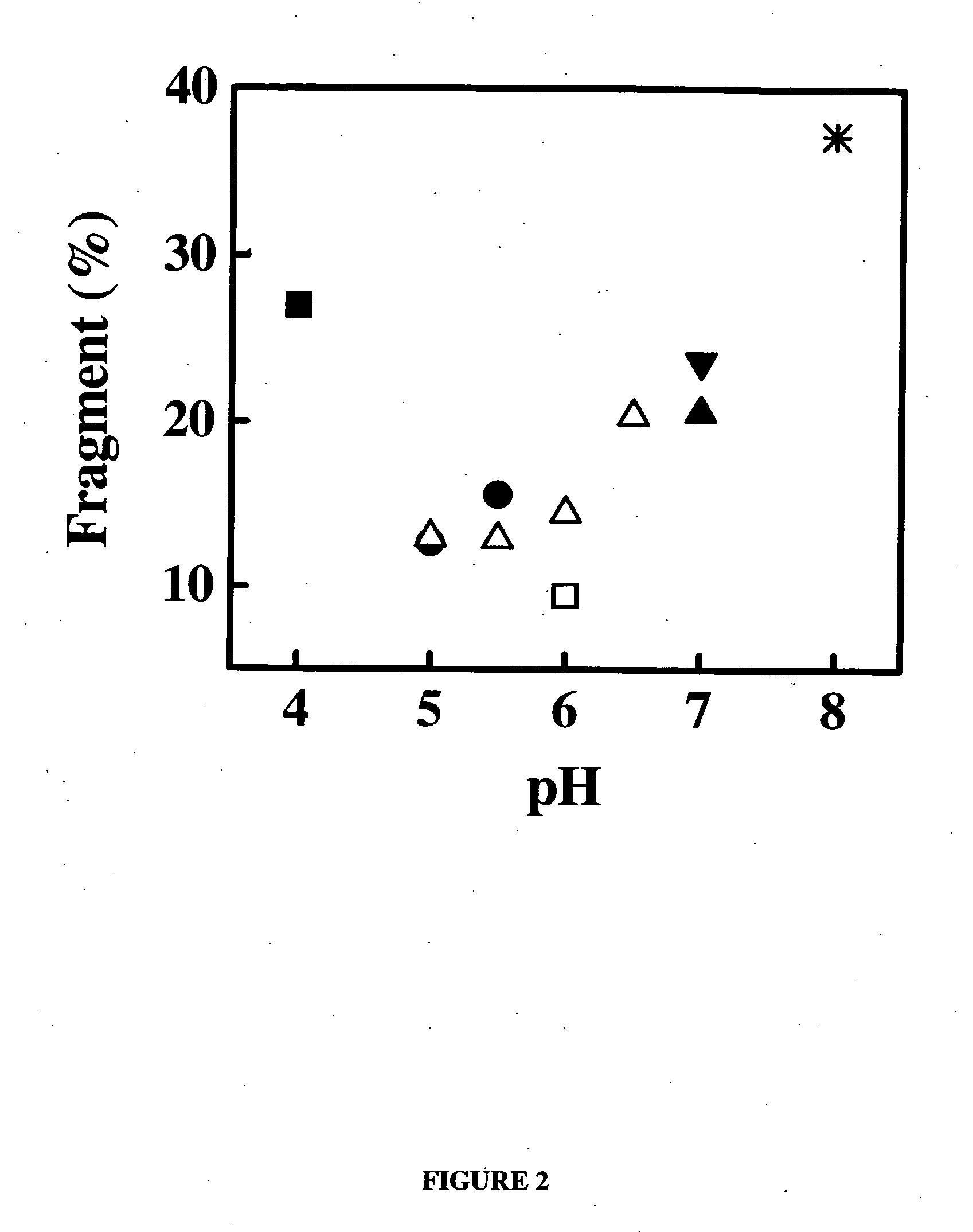
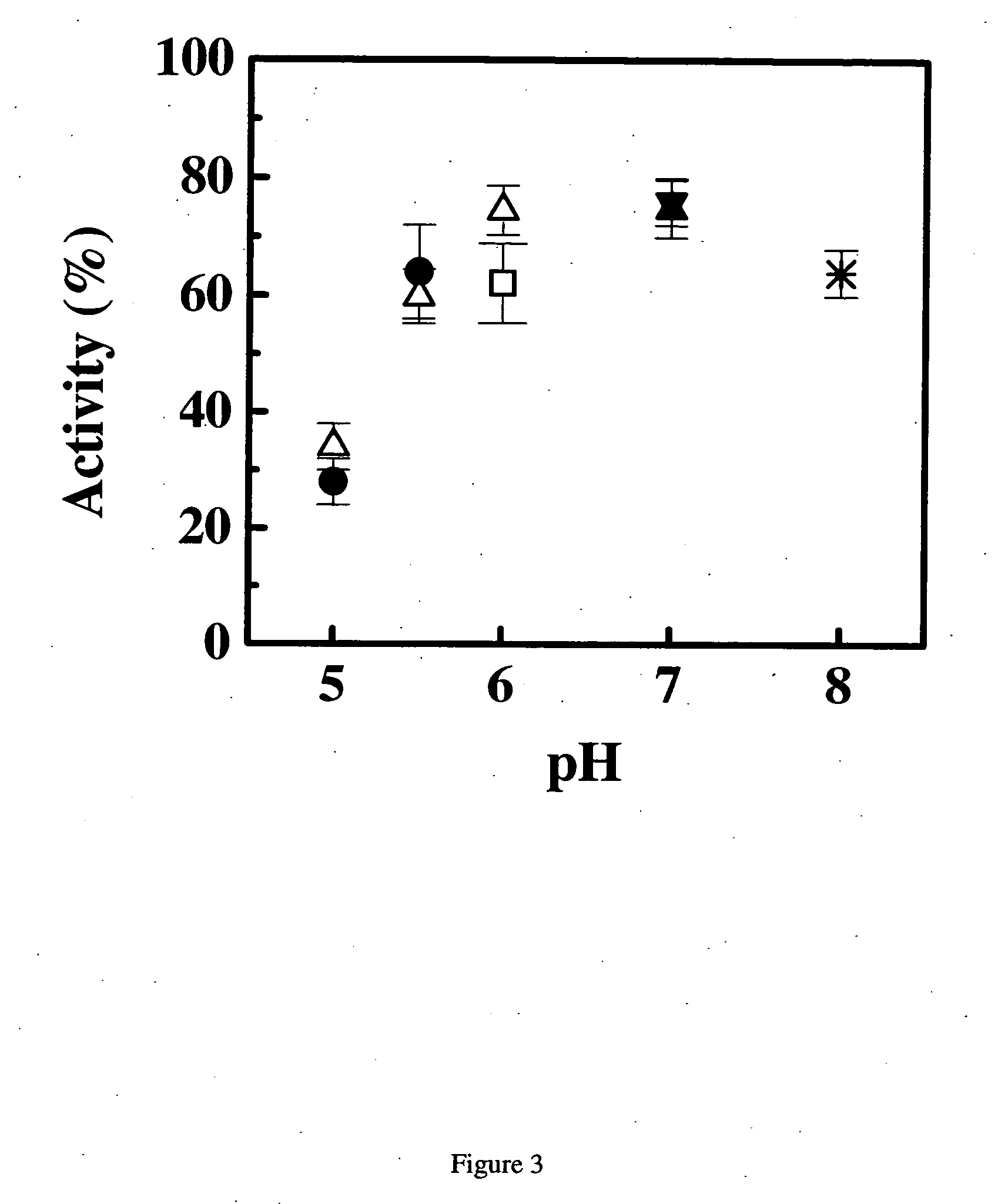
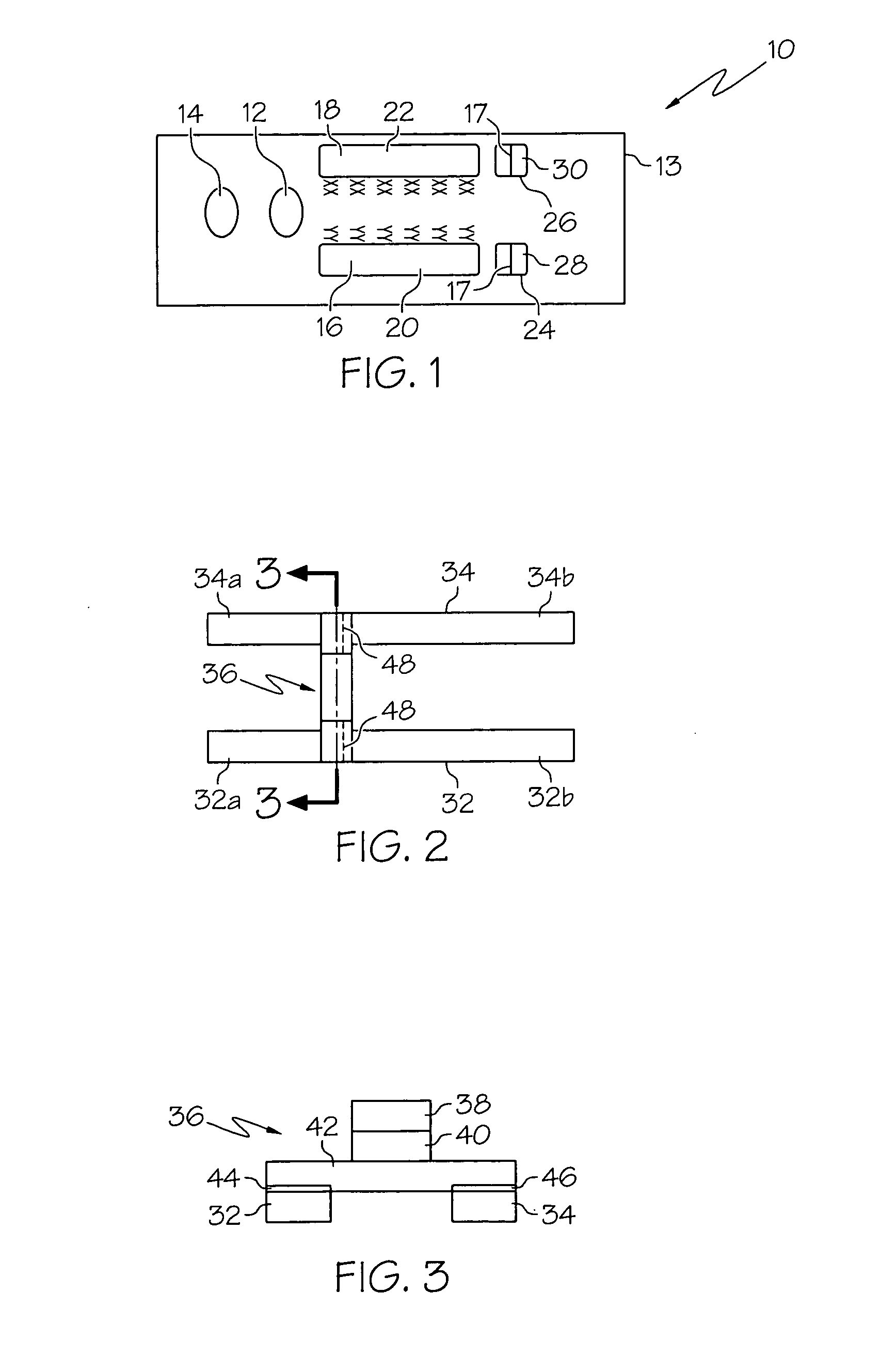
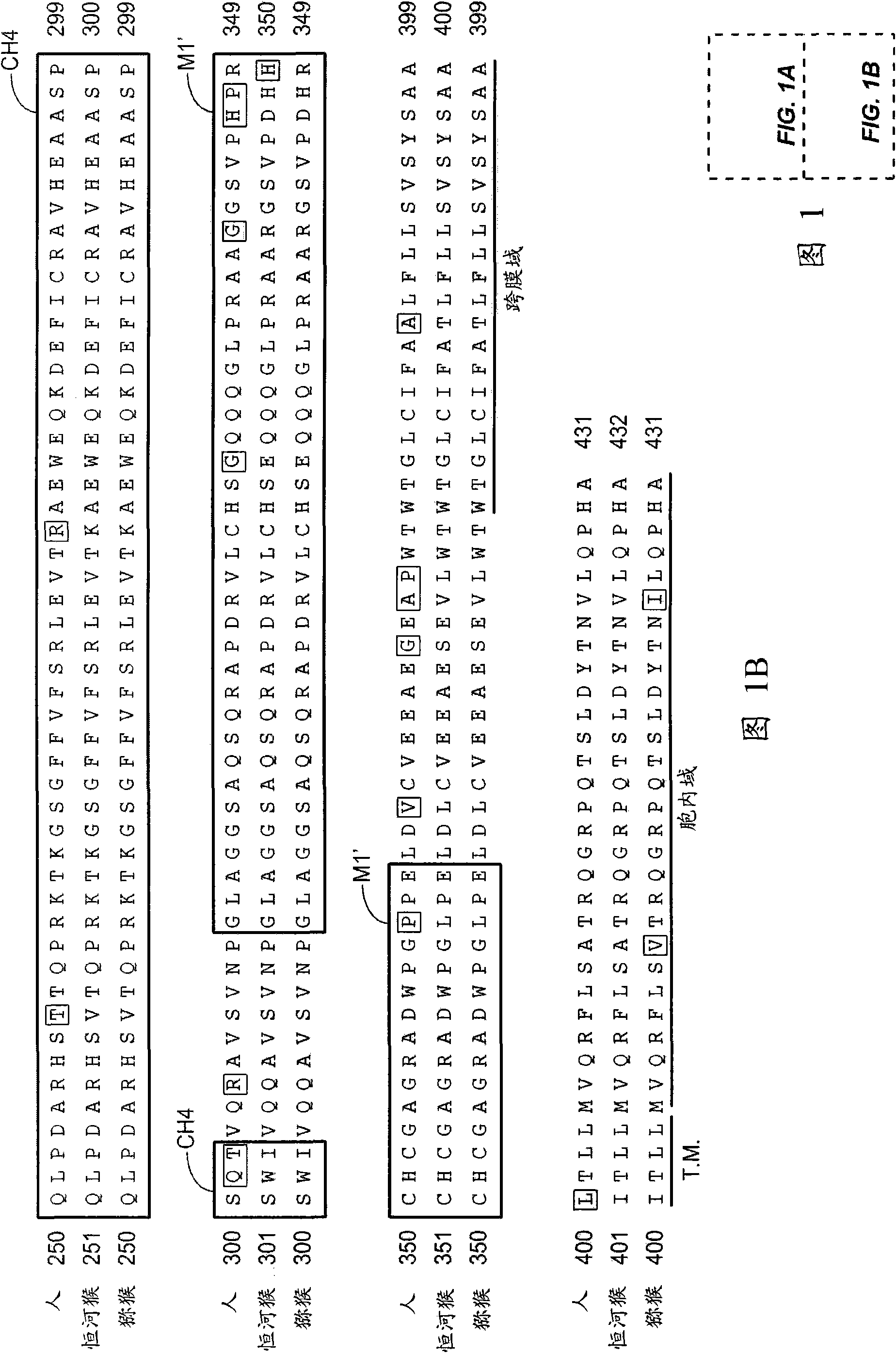
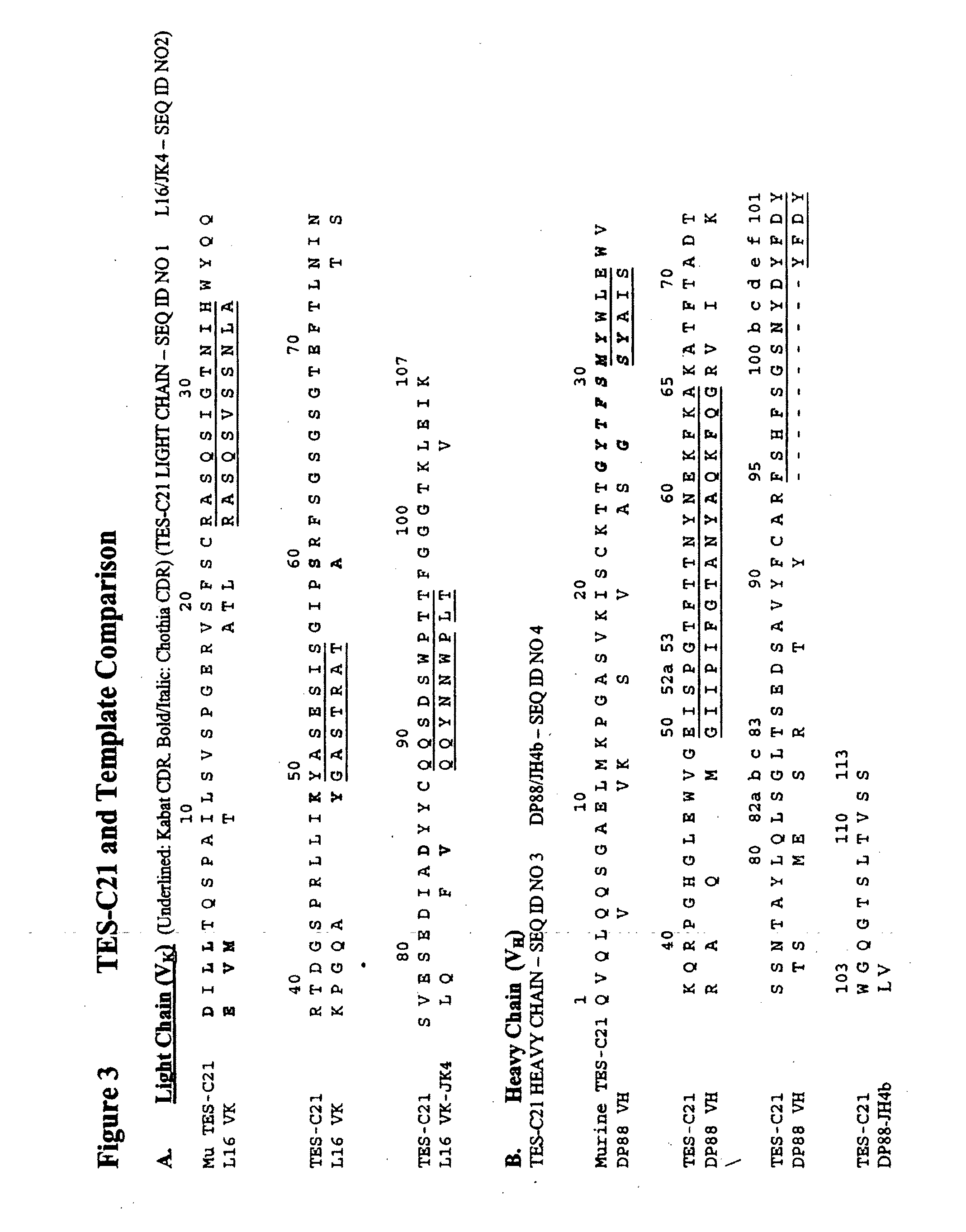
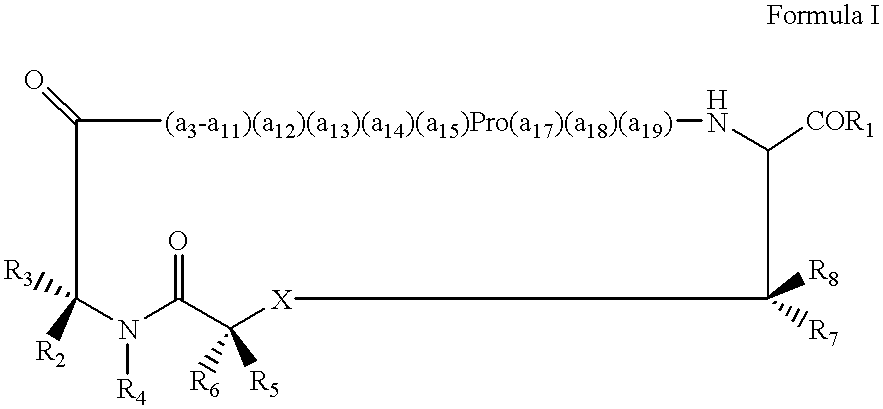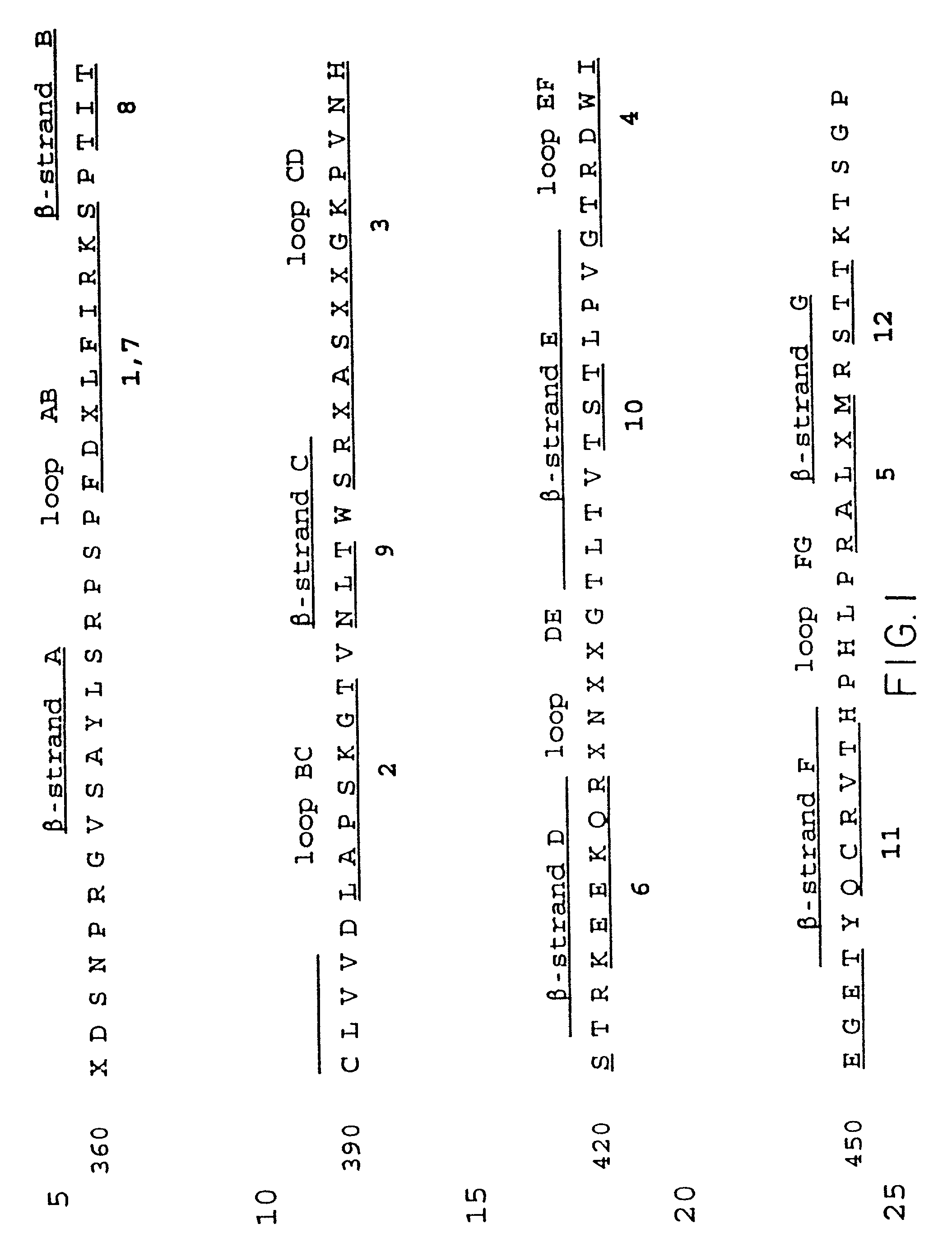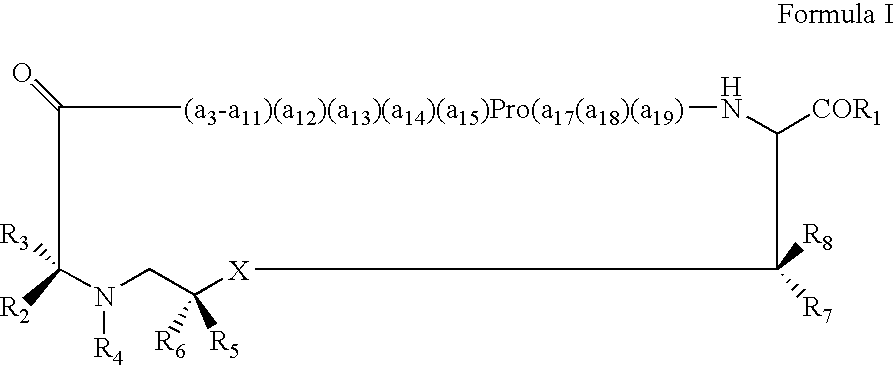Patents
Literature
43 results about "Anti-IgE Antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
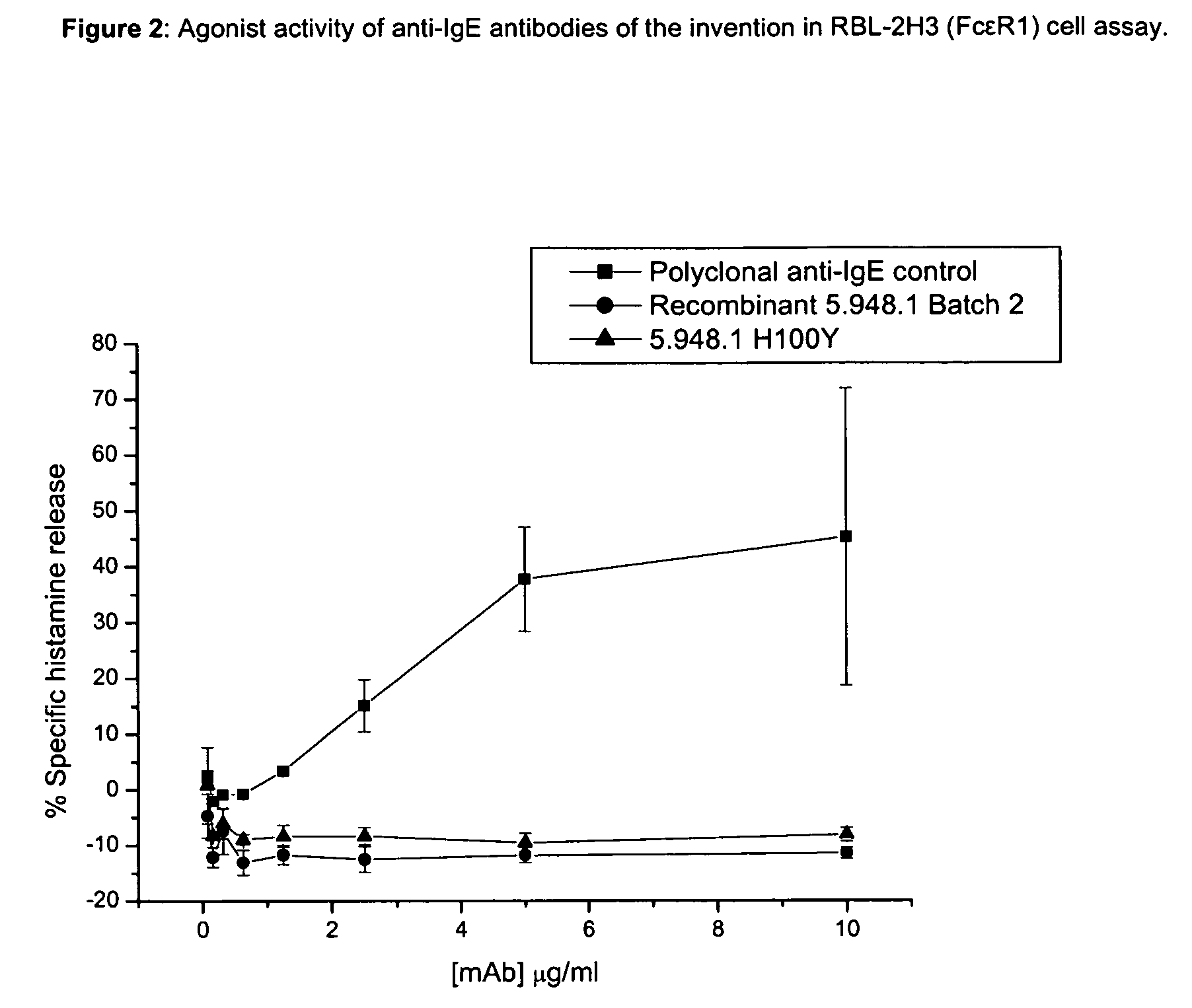
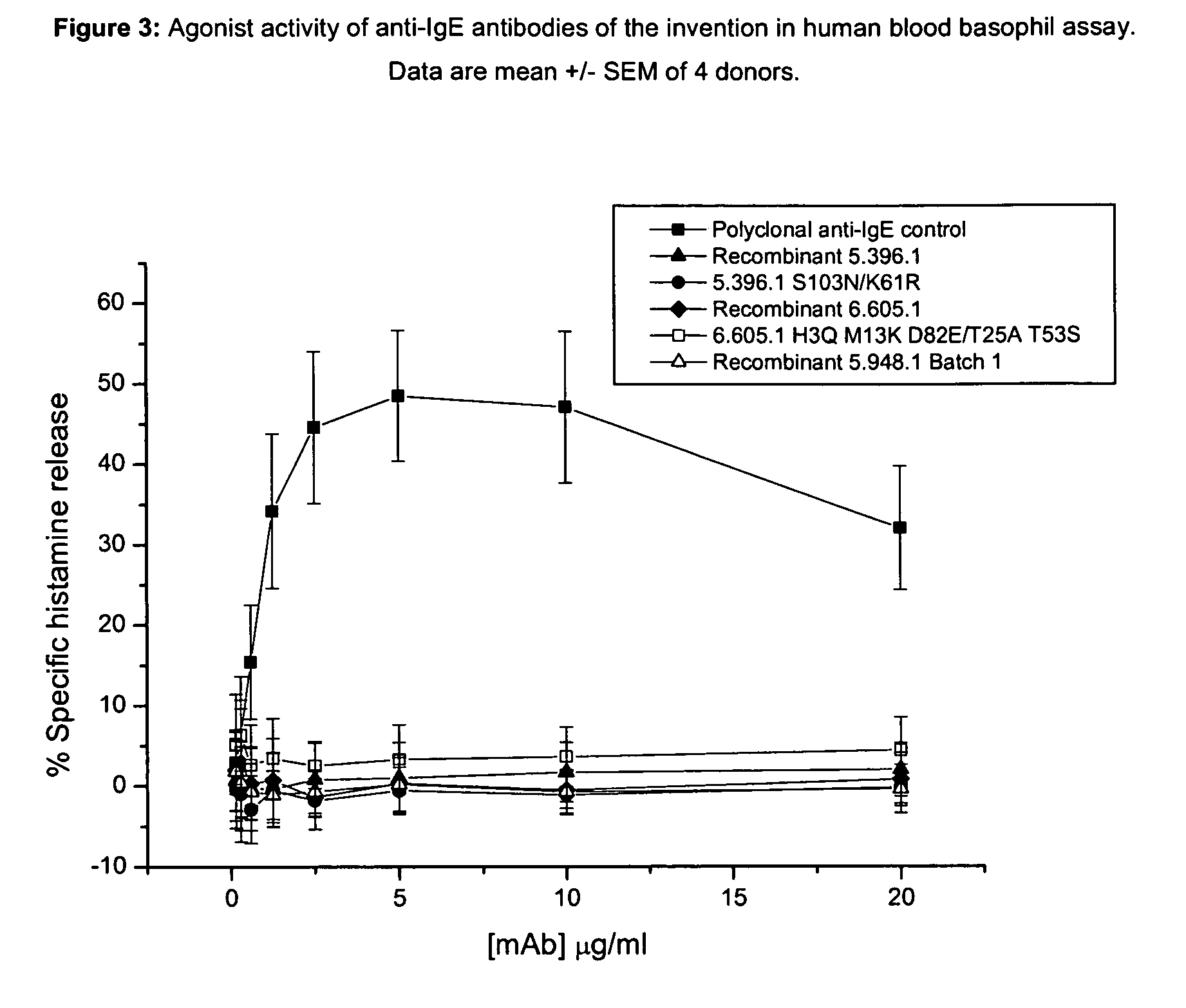
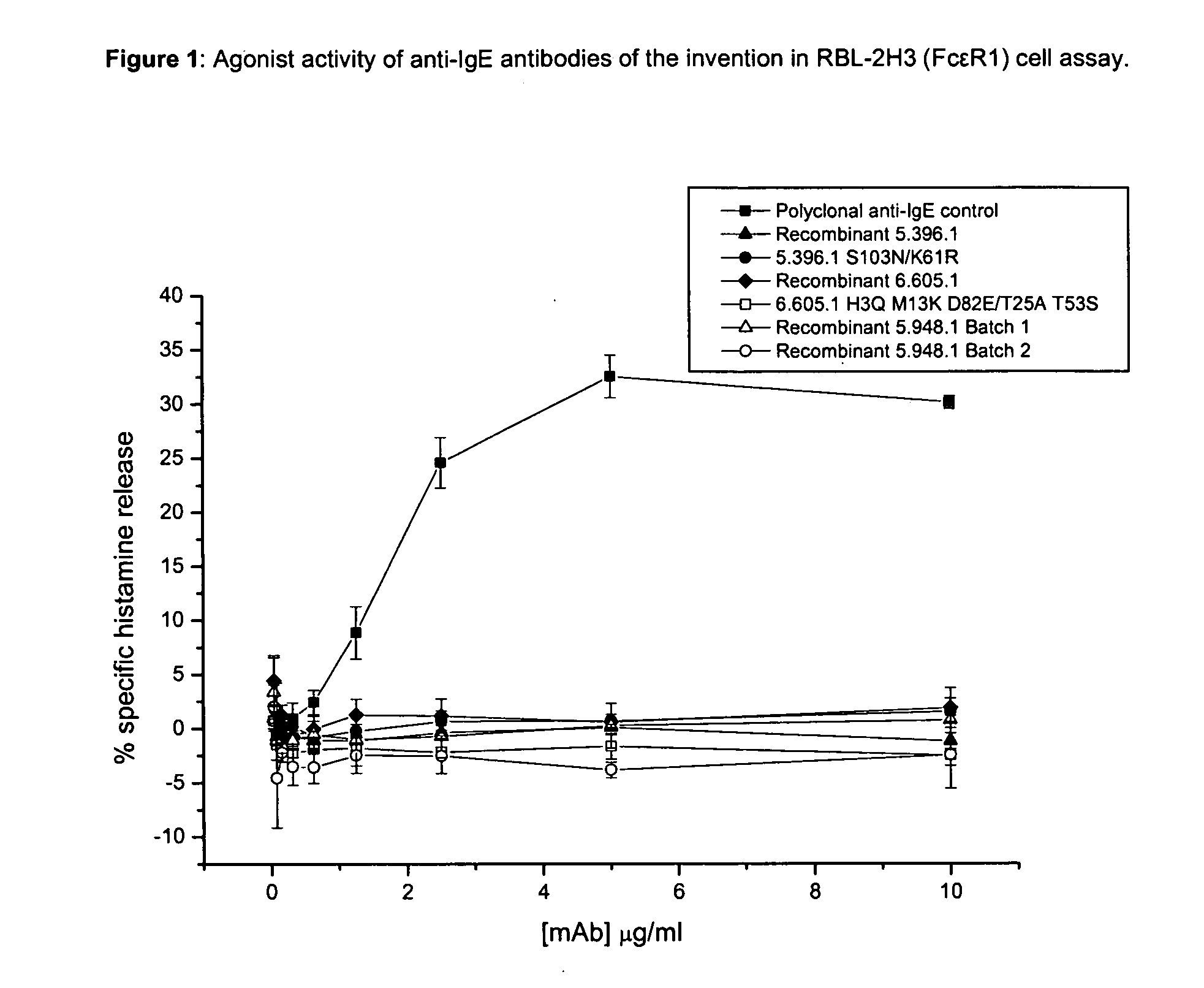
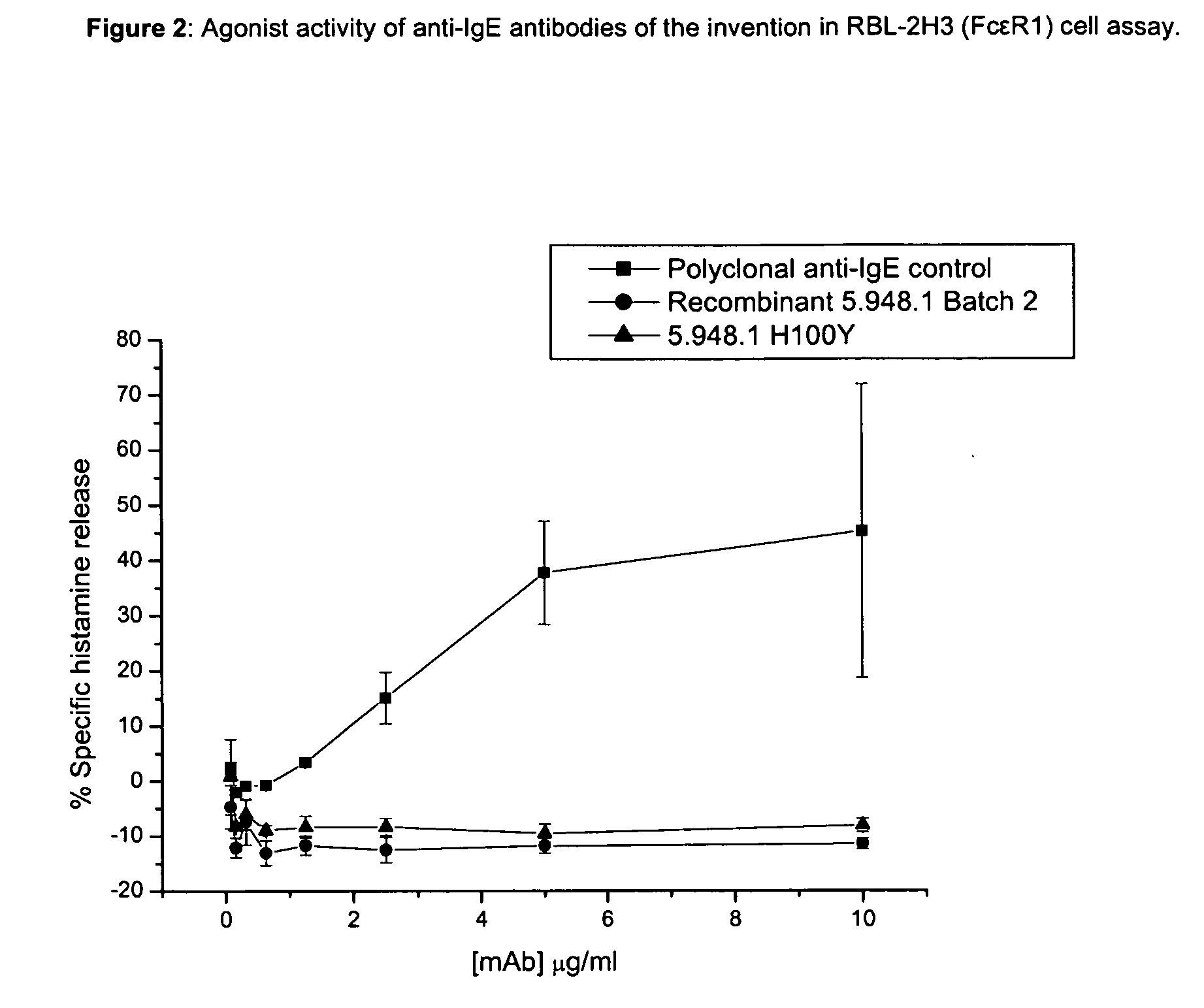
Unlike an ordinary anti-IgE antibody, it does not bind to IgE that is already bound by the high affinity IgE receptor (FcεRI) on the surface of mast cells, basophils, and antigen-presenting dendritic cells. Medical uses Allergic asthma. Omalizumab is used to treat people with severe ...
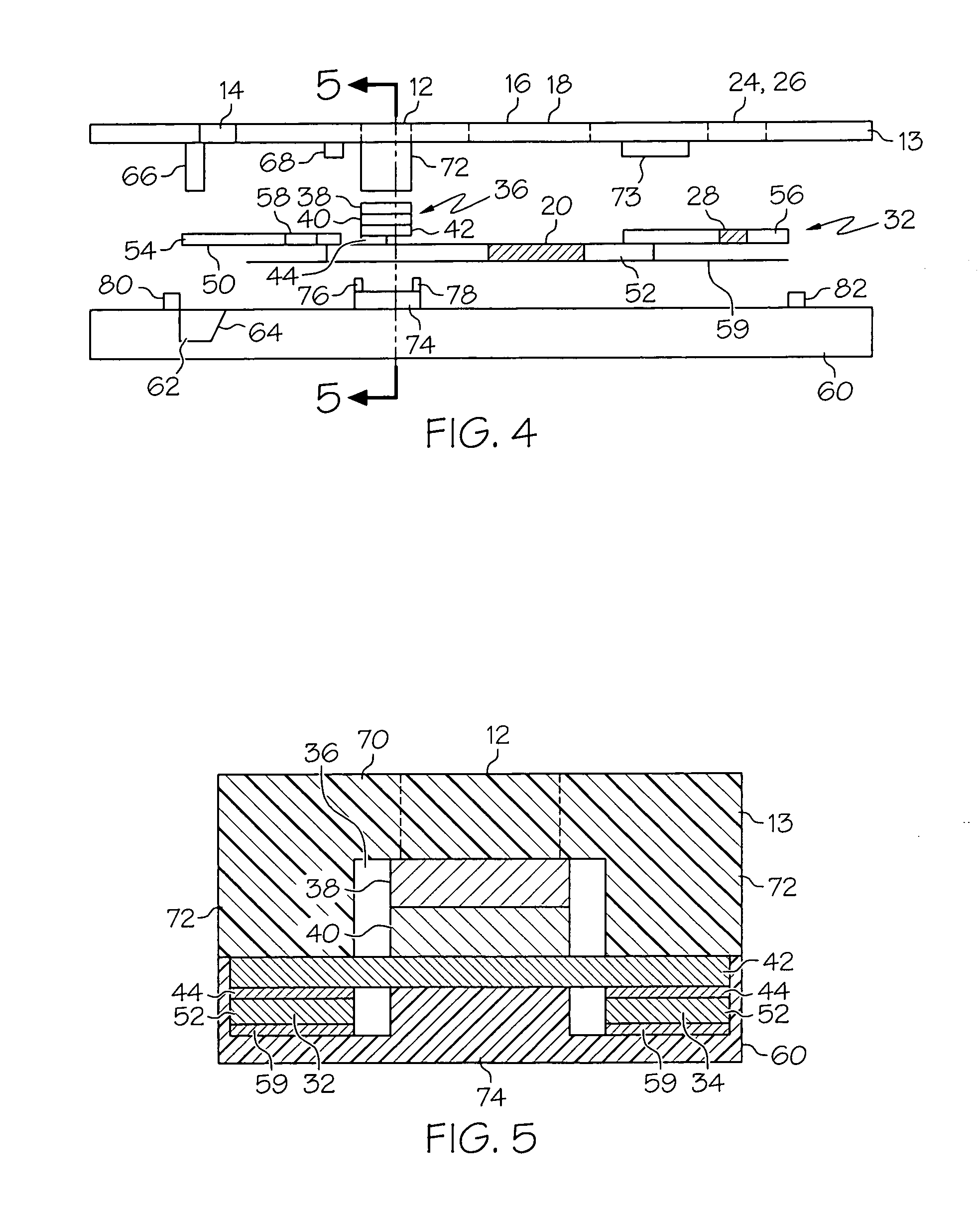
Methods of treating IgE-mediated disorders comprising the administration of high concentration anti-IgE antibody formulations
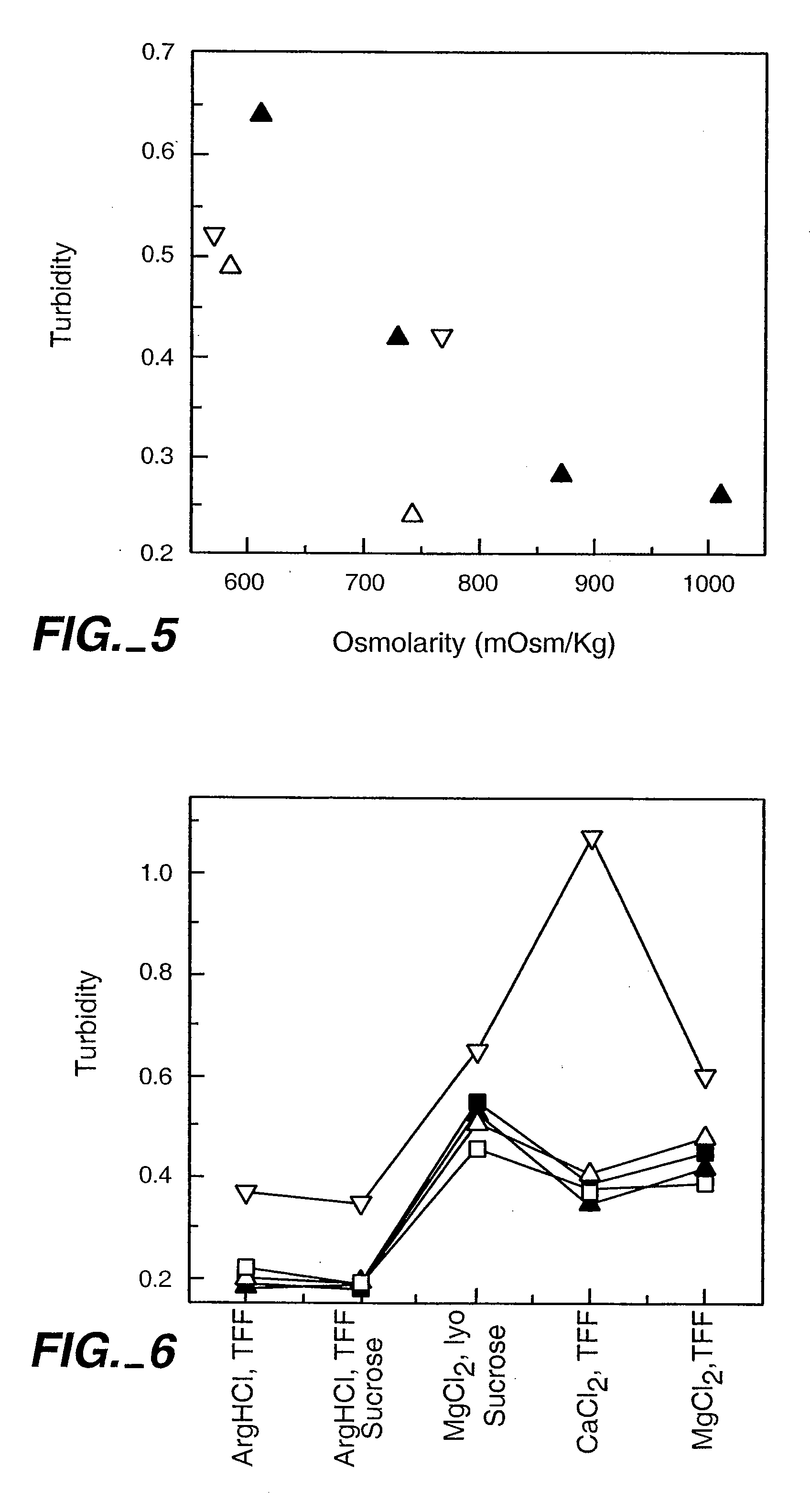
InactiveUS20050158303A1Speed up concentrationReduce turbidityOrganic active ingredientsBiocideIge mediatedAnti-IgE Antibody
The present application relates to methods of treating IgE-mediated disorders comprising the administration of highly concentrated anti-IgE antibody formulations with reduced viscosity that are stable, relatively isotonic and are of low turbidity. The formulations are particularly suitable for subcutaneous administration.
Owner:GENENTECH INC +1
Two step lateral flow assay methods and devices
ActiveUS20070020768A1Easy to optimizeEasy to detectEnzymologyDisease diagnosisAntigenLateral flow immunoassay
Lateral flow assay devices and methods for detecting a first member of a specific binding pair in a sample which comprises a plurality of nonspecific binding pair members are adapted for two step determinations. In one embodiment, a two step lateral flow assay method for identifying IgE antibodies in a sample comprises applying a sample to a sample port of a device, wherein the device is adapted to deliver the sample to a lateral flow matrix having a plurality of IgE antigen species immobilized at respective positions at a first location The two step method further comprises allowing the sample to travel along the lateral flow matrix through the immobilized plurality of IgE antigen species to a second location downstream of the first location, applying liquid buffer to the lateral flow matrix to mobilize labeled reagent which is adapted to bind anti-IgE antibody and is dried on the lateral flow matrix at a location upstream of the delivery of the filtered sample to the lateral flow matrix, and allowing labeled reagent mobilized by the liquid buffer to travel along the lateral flow matrix through the immobilized plurality of IgE antigen species to a location downstream of the first location. Further embodiments comprise additional lateral flow immunoassay devices and methods for identifying IgE antibodies in a sample.
Owner:PHADIA AB
Methods of treating IgE-mediated disorders comprising the administration of high concentration anti-IgE antibody formulations
The present application relates to methods of treating IgE-mediated disorders comprising the administration of highly concentrated anti-IgE antibody formulations with reduced viscosity that are stable, relatively isotonic and are of low turbidity. The formulations are particularly suitable for subcutaneous administration.
Owner:GENENTECH INC
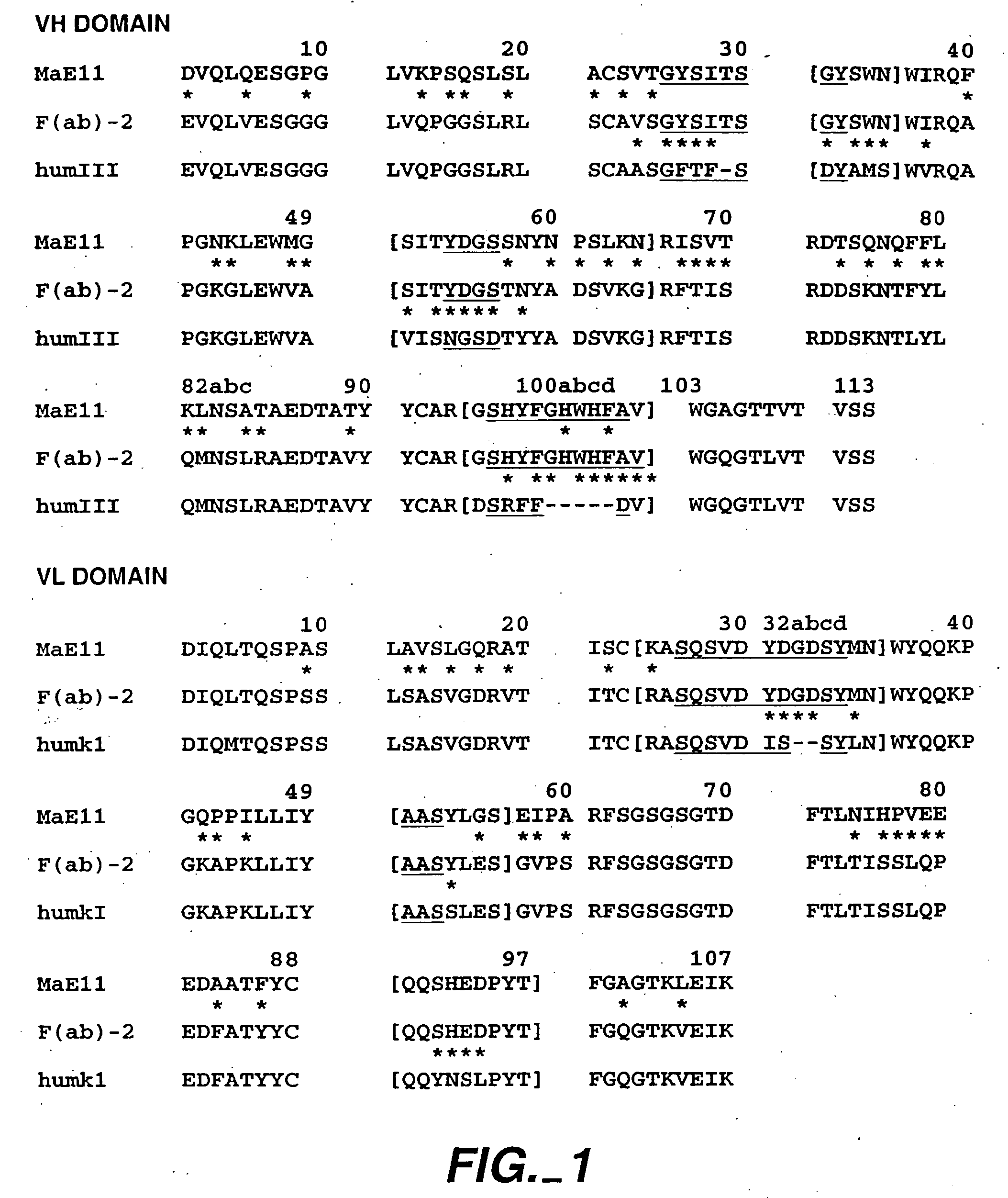
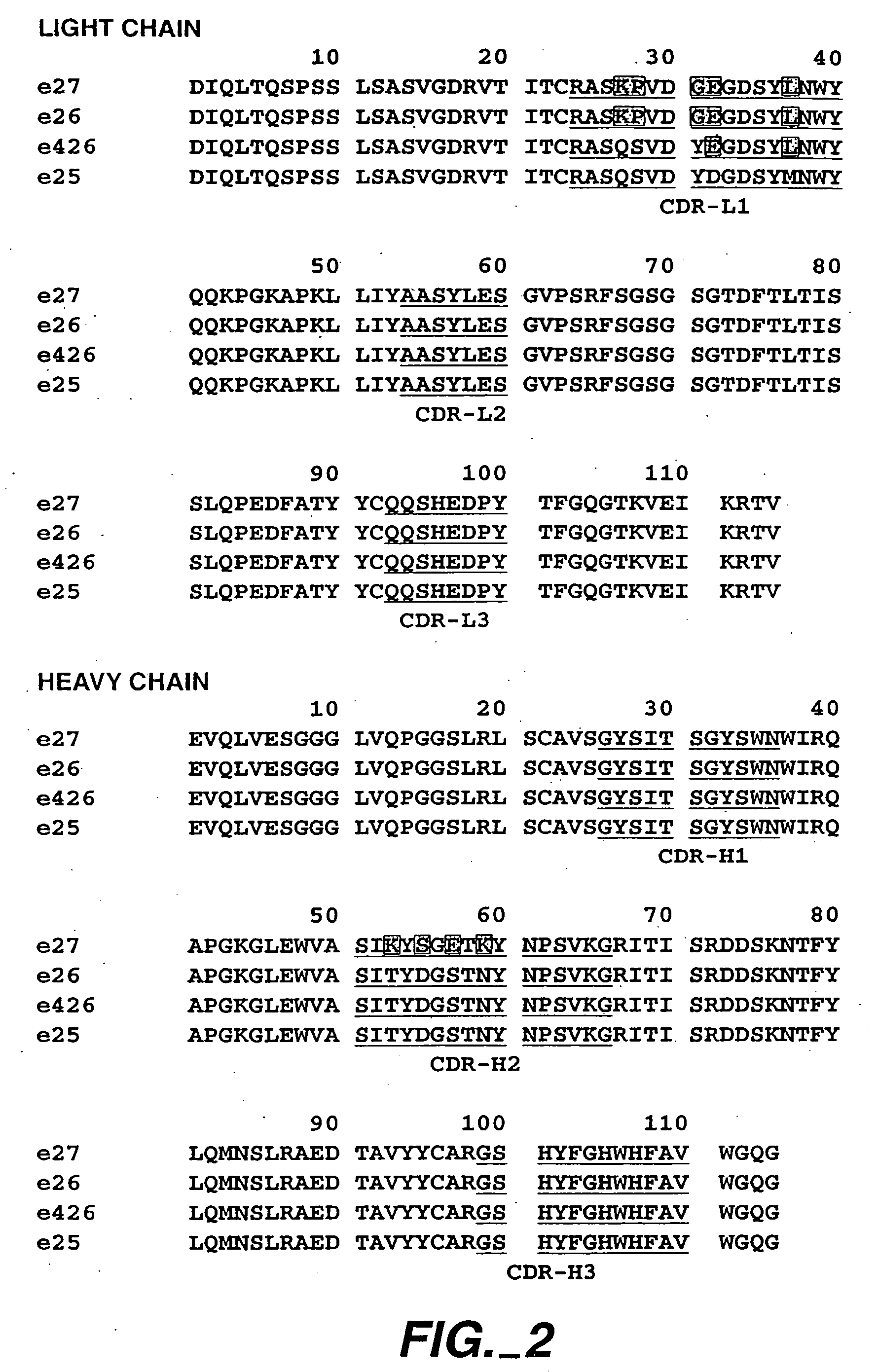
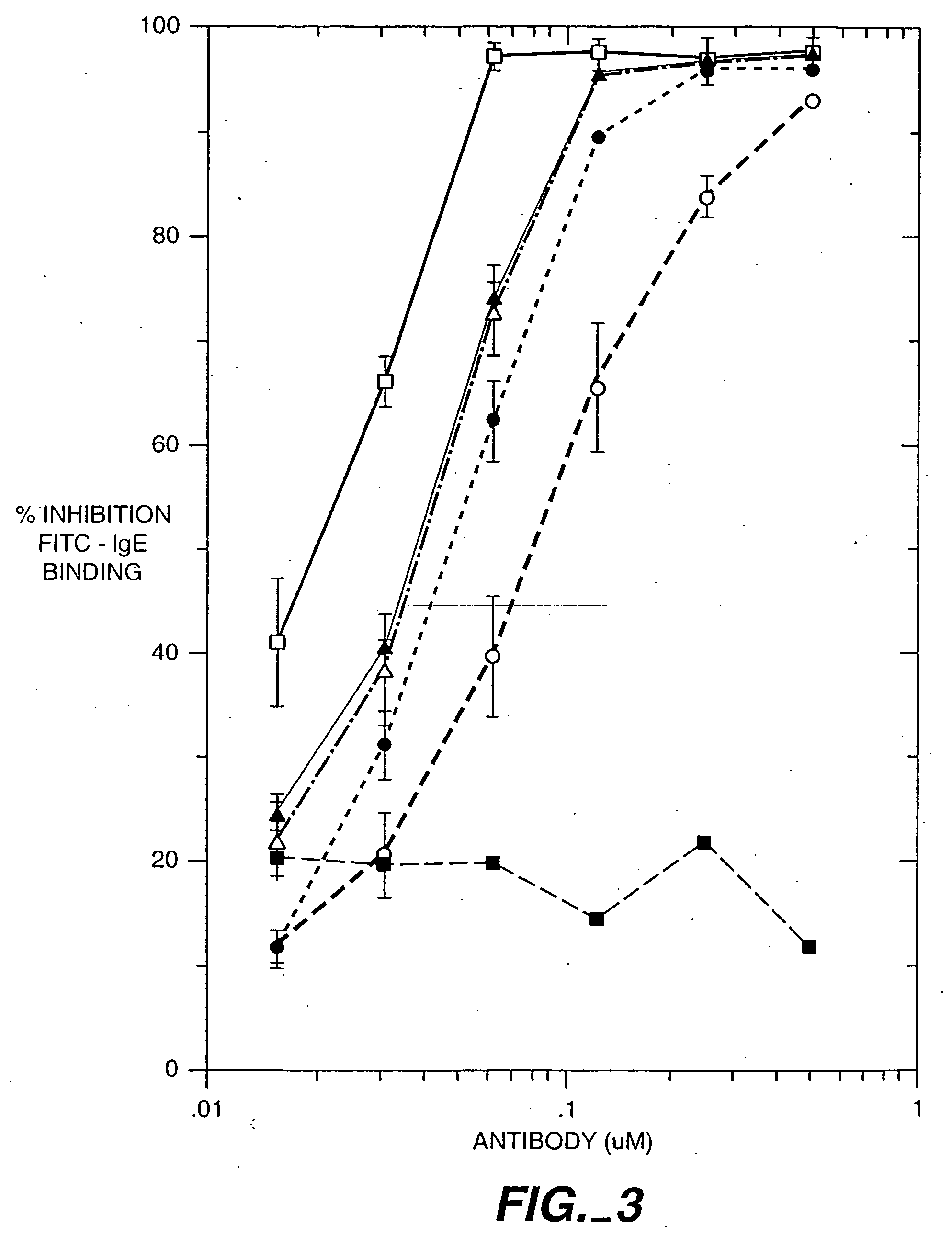
Anti-IgE antibodies
InactiveUS20080003218A1Reducing and inhibiting IgE-mediated productionAnimal cellsSugar derivativesIsomerizationBacteriophage
The present invention relates to a method for adjusting the affinity of a polypeptide to a target molecule by a combination of steps, including: (1) the identification of aspartyl residues which are prone to isomerization; (2) the substitution of alternative residues and screening the resulting mutants for affinity against the target molecule. In a preferred embodiment, the method of subtituting residues is affinity maturation with phage display (AMPD). In a further preferred embodiment the polypeptide is an antibody and the target molecule is an antigen. In a further preferred embodiment, the antibody is anti-IgE and the target molecule is IgE. In another embodiment, the invention relates to an anti-IgE antibody having improved affinity to IgE.
Owner:GENENTECH INC
METHODS OF TREATING IgE-MEDIATED DISORDERS COMPRISING THE ADMINISTRATION OF HIGH CONCENTRATION ANTI-IgE ANTIBODY FORMULATIONS
The present application relates to methods of treating IgE-mediated disorders comprising the administration of highly concentrated anti-IgE antibody formulations with reduced viscosity that are stable, relatively isotonic and are of low turbidity. The formulations are particularly suitable for subcutaneous administration.
Owner:GENENTECH INC +1
Anti-IgE antibodies
The present invention relates to novel human antibodies specifically directed against human immunoglobulin E (anti-IgE). The present invention also relates to pharmaceutical compositions and methods for treating asthma, in particular allergic asthma, as well as other IgE-mediated disorders including allergic rhinitis and food allergies.
Owner:PFIZER INC +1
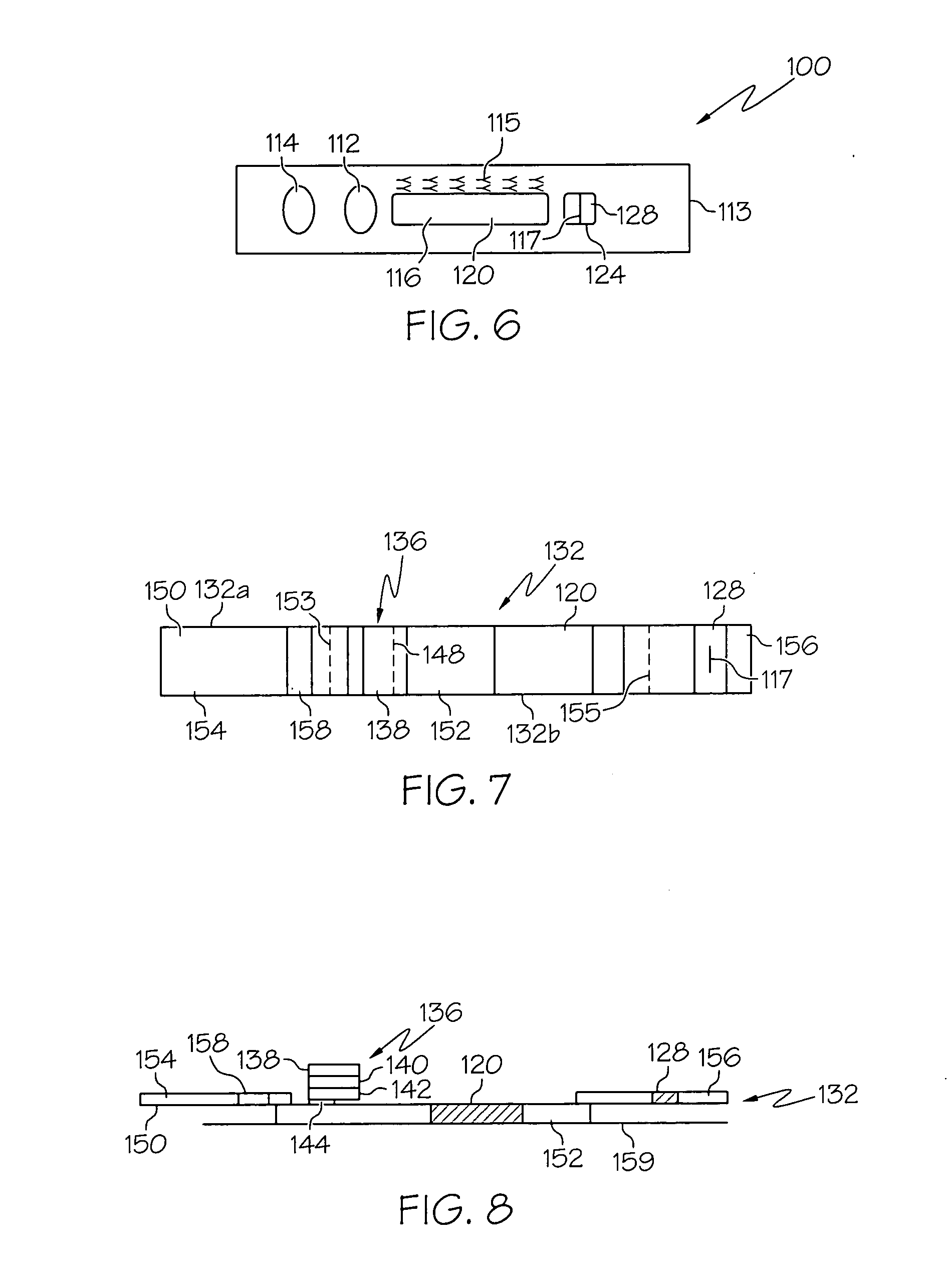
Two step lateral flow assay methods and devices
ActiveUS7871781B2Assist in diagnosing allergy in an individualSpeed up the flowBioreactor/fermenter combinationsBiological substance pretreatmentsAntigenAnalyte
Lateral flow assay devices and methods for detecting an analyte in a sample which comprises a plurality of nonspecific binding pair members are adapted for two step determinations. In one embodiment, a method for identifying IgE antibodies in a sample comprises applying a sample to a sample port of a device, wherein the device is adapted to deliver the sample to a lateral flow matrix having a plurality of IgE antigen species immobilized at respective positions at a first location, allowing the sample to travel along the lateral flow matrix through the immobilized plurality of IgE antigen species to a second location downstream of the first location and allowing sample to dissolve a water-soluble mark, applying liquid buffer to the lateral flow matrix to mobilize labeled reagent which is adapted to bind anti-IgE antibody and which is dried on the lateral flow matrix at a location upstream of the sample delivery location, and allowing labeled reagent to bind any IgE antibody bound to the immobilized IgE antigen species therein.
Owner:PHADIA AB
Method for treating IgE-mediated disorders
InactiveUS7157085B2Reducing and inhibiting IgE-mediated productionSugar derivativesMicrobiological testing/measurementDiseaseIsomerization
The present invention relates to a method for adjusting the affinity of a polypeptide to a target molecule by a combination of steps, including: (1) the identification of aspartyl residues which are prone to isomerization; (2) the substitution of alternative residues and screening the resulting mutants for affinity against the target molecule. In a preferred embodiment, the method of subtituting residues is affinity maturation with phage display (AMPD). In a further preferred embodiment the polypeptide is an antibody and the target molecule is an antigen. In a further preferred embodiment, the antibody is anti-IgE and the target molecule is IgE. In another embodiment, the invention relates to an anti-IgE antibody having improved affinity to IgE.
Owner:GENENTECH INC
Methods of treating IgE-associated disorders and compositions for use therein
InactiveUS20060013811A1Avoid anaphylactic shockHigh activityOrganic active ingredientsPeptide/protein ingredientsAntigenDisease
The present invention provides methods of treating IgE-associated disorders and compositions for use therein. The methods are particularly useful in treatment of allergies and allergy-related disorders. The methods generally comprise administering an IgE inhibitor (such as anti-IgE antibody) and an antigen and / or immunostimulatory polynucleotide sequence (ISS). These combination methods offer significant advantages, such as allowing more aggressive therapy while reducing unwanted side effects, such as anaphylaxis.
Owner:DYNAVAX TECH CORP
Method of treating allergic disorders
InactiveUS20010033842A1Hybrid immunoglobulinsPeptide/protein ingredientsDiseaseAnaphylactic reaction
The present invention describes IgE antagonists (including variant anti-IgE antibodies) and their use in diagnosis, therapy or prophylaxis of allergic and other IgE-mediated disorders, including asthma, food allergies, hypersensitivity and anaphylactic reactions.
Owner:GENENTECH INC
Anti-IgE antibodies
The present invention relates to novel human antibodies specifically directed against human immunoglobulin E (anti-IgE). The present invention also relates to pharmaceutical compositions and methods for treating asthma, in particular allergic asthma, as well as other IgE-mediated disorders including allergic rhinitis and food allergies.
Owner:PFIZER INC +1
Assays for detecting antibodies specific to therapeutic Anti-ige antibodies and their use in anaphylaxis
ActiveUS20110183363A1Low affinityImmunoglobulins against animals/humansDisease diagnosisAnaphylactic reactionAntibody
The invention provides methods and reagents useful for detecting anti-drug antibodies of IgE isotype to therapeutic anti-IgE antibodies, and methods for assessing risk of anaphylaxis to administration of a therapeutic anti-IgE antibody.
Owner:GENENTECH INC
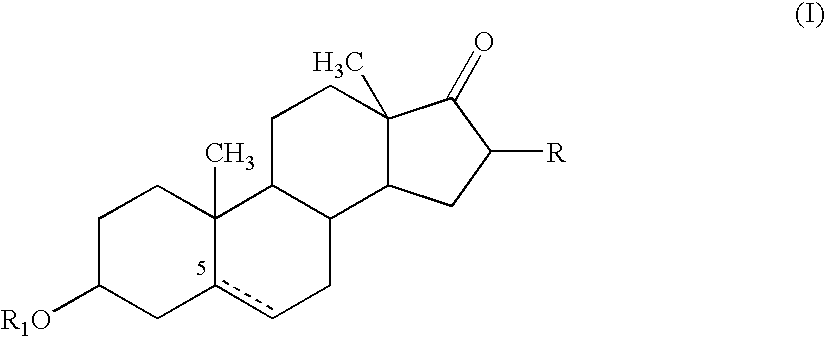
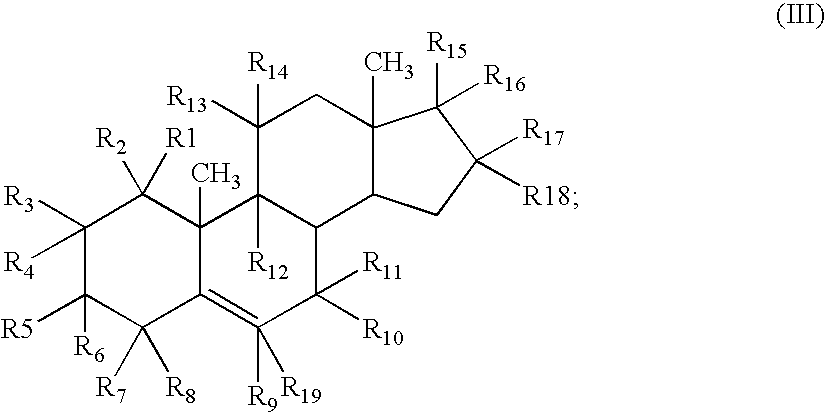
Combination of dehydroepiandrosterone or dehydroepiandrosterone-sulfate with an anti-IgE antibody for treatment of asthma or chronic obstructive pulmonary disease
A pharmaceutical or veterinary composition, comprises a first active agent selected from a dehydroepiandrosterone and / or dehydroepiandrosterone-sulfate, or a salt thereof, and a second active agent comprising an anti-IgE antibody for the treatment of asthma, chronic obstructive pulmonary disease, or other respiratory diseases. The composition is provided in various formulations and in the form of a kit. The products of this patent are applied to the prophylaxis and treatment of asthma, chronic obstructive pulmonary disease, or other respiratory diseases.
Owner:EPIGENESIS PHARMA LLC
Methods for treatment of allergic asthma
InactiveUS20070020256A1Reducing late asthmatic responseReducing early asthmatic responseAntibody ingredientsImmunoglobulinsAllergic asthmaAntagonist
Methods of treatment of allergic asthma with IgE antagonists, including anti-IgE antibodies, IgE variants, peptide antagonists, peptidomimetics and other small molecules, are described.
Owner:GENENTECH INC
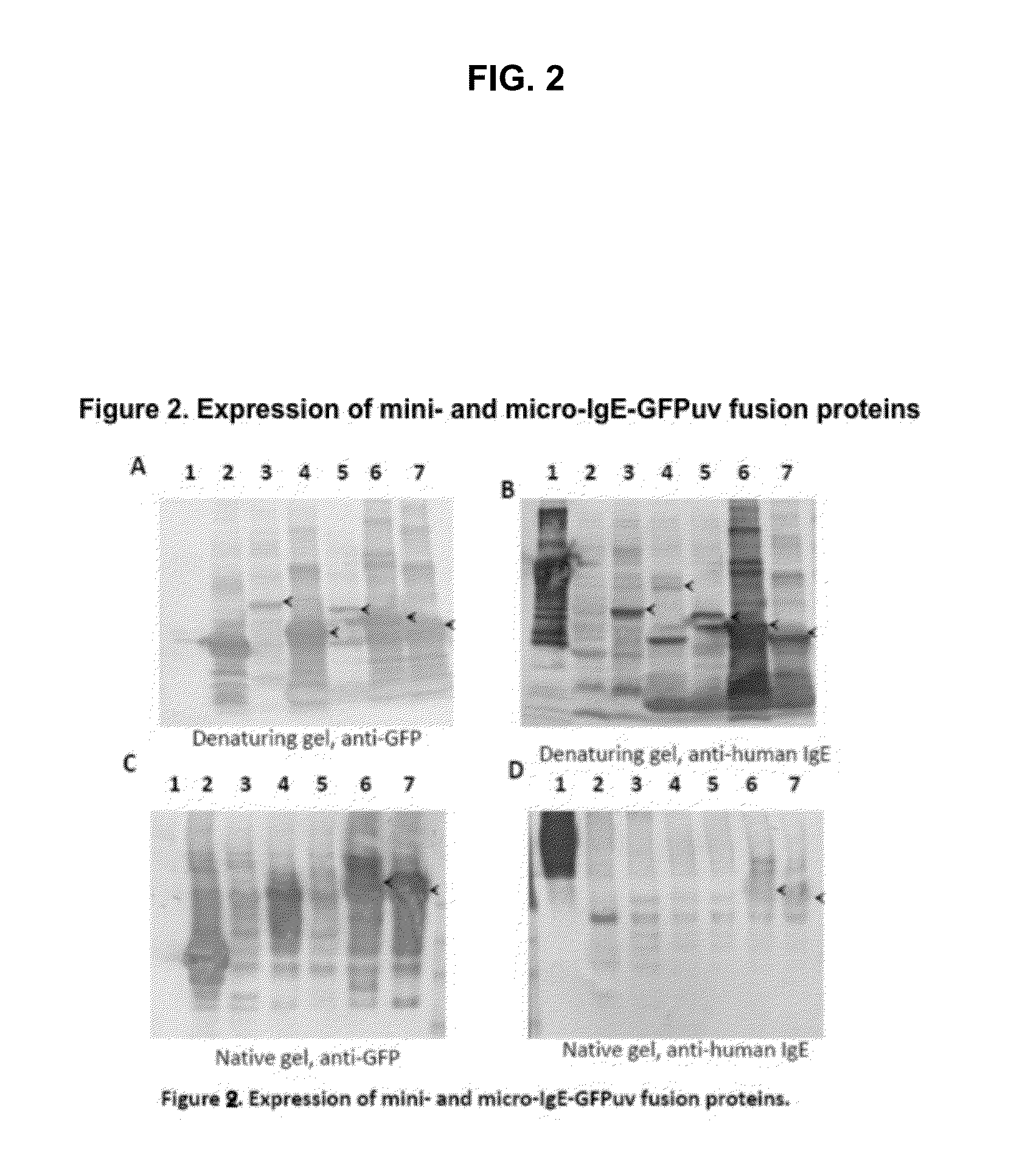
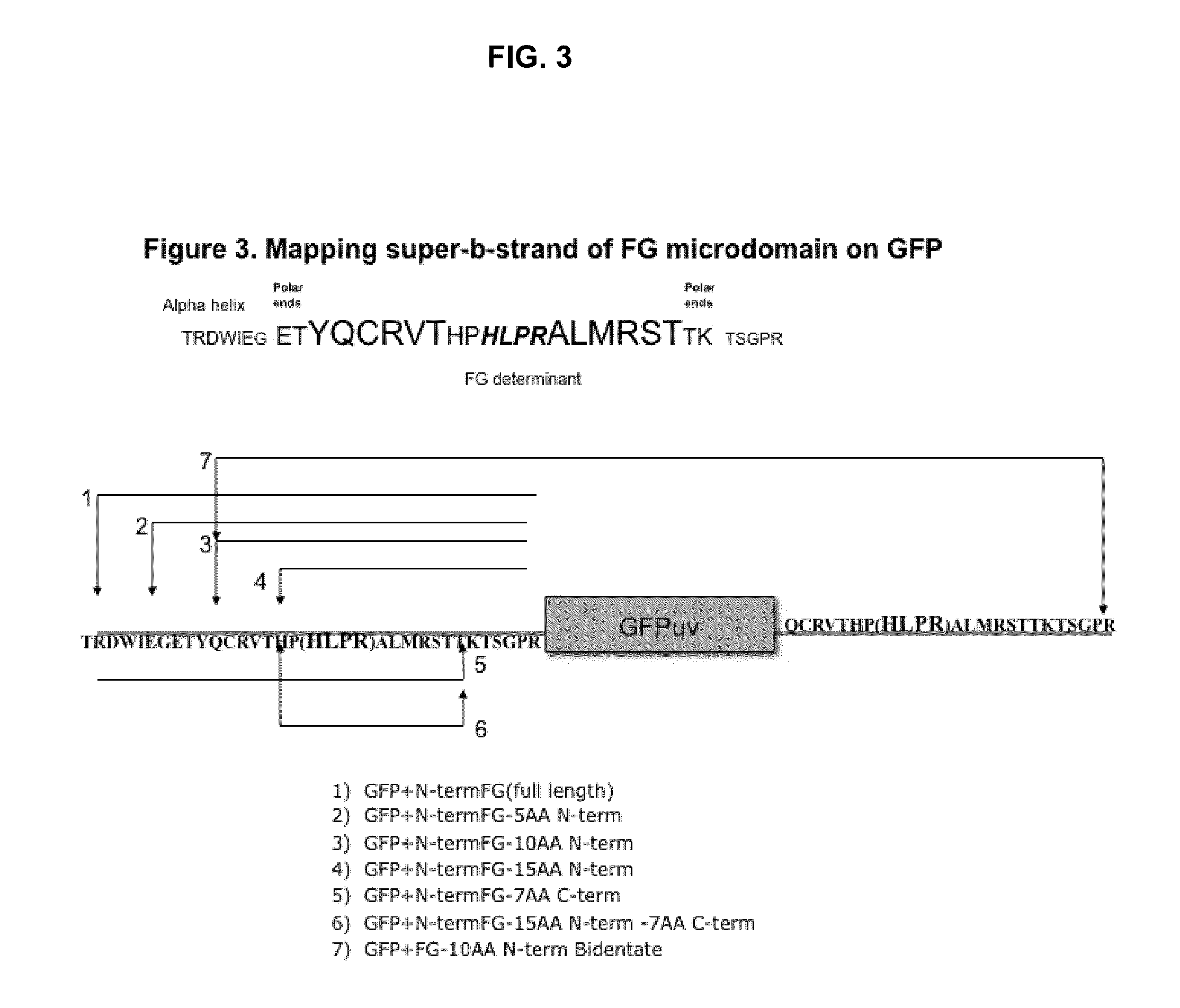
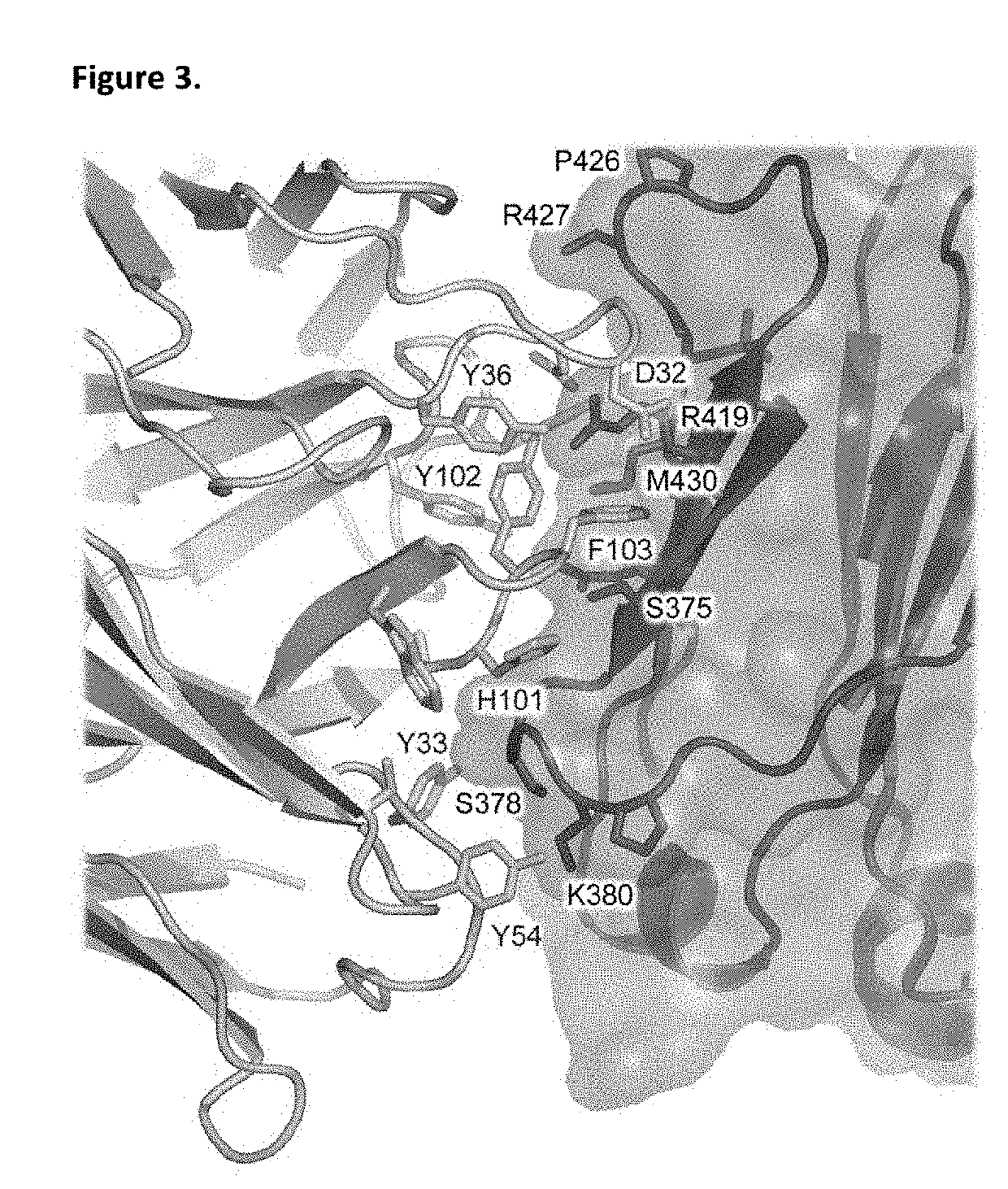
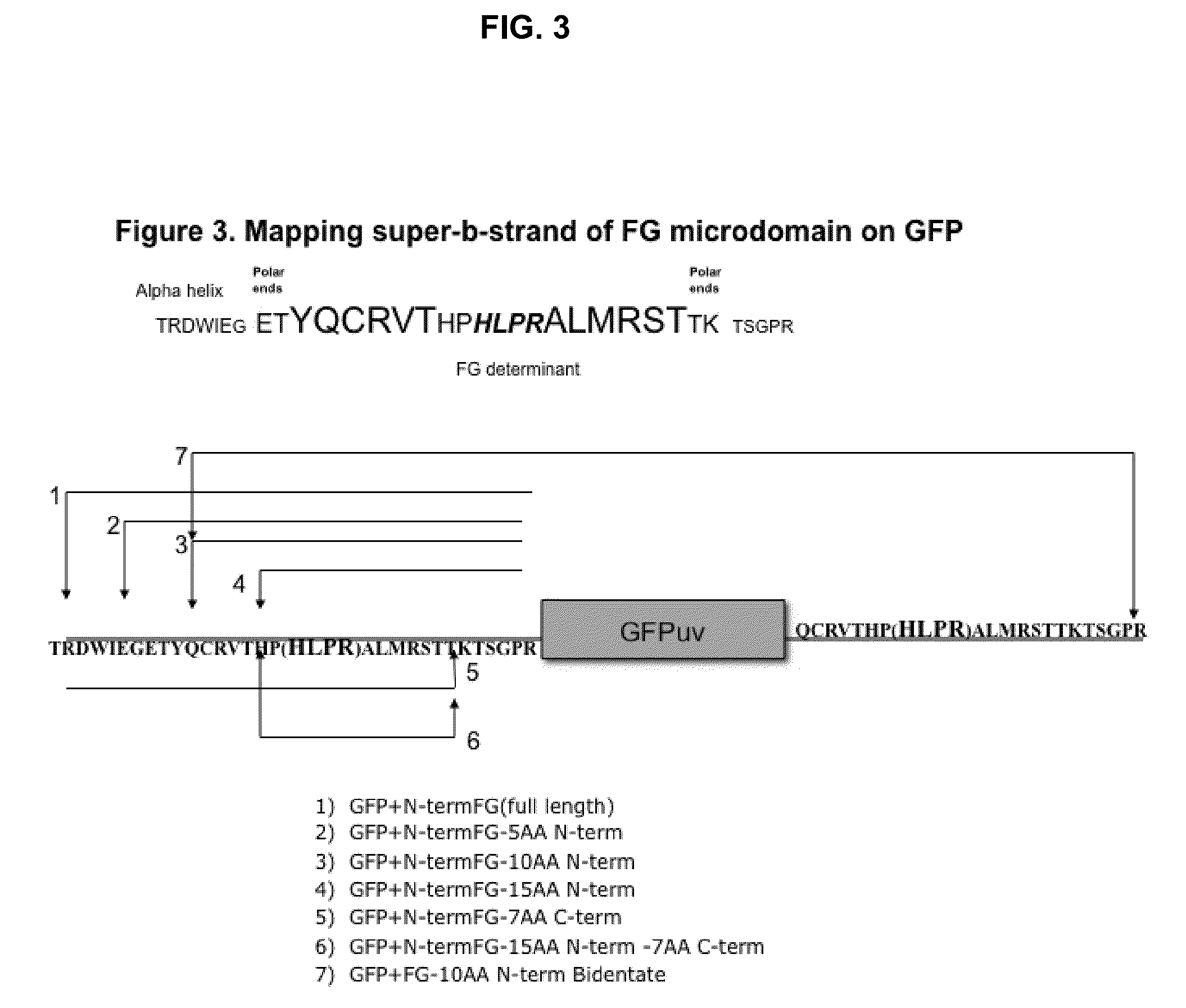
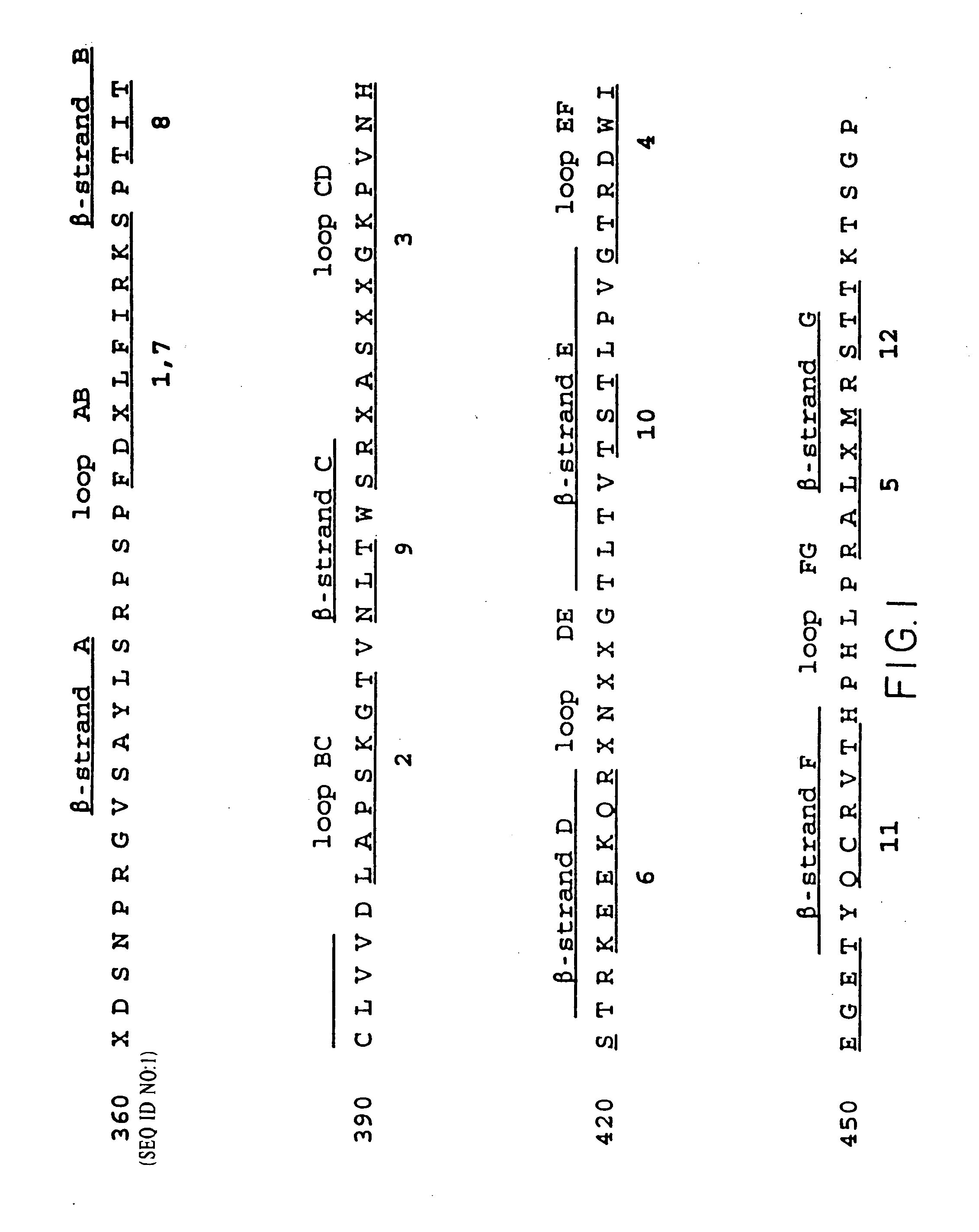
Displaying native human IgE neutralizing FceRla-contacting IgE B-cell epitopes by constraining super beta(b)-strands and cystine knots on thermostable protein scaffold
Vaccine displaying native antigenic loops of immunoglobulin E is critical for eliciting neutralizing anti-IgE antibodies. The embodiment of the invention enables the display of native antigenic IgE receptor-contacting loops as IgE B-cell vaccines via three steps of constraining methods. The loops of multiple antigenic B-cell epitopes can be molecularly grafted in, and conformationally constrained by the energy favorable flanking beta (b)-stands, i.e., the super b-strands identified in this invention. The constrained loops can be further stabilized in replacing a selective loop within the cystine knot peptide. These dual constrained antigenic loops are then integrated onto thermostable protein scaffolds, folded in the oxidative milieu that provides further conformational constraint and high yield.
Owner:CHEN SWEY SHEN
ANTI-IgE ANTIBODIES
ActiveUS20190144565A1Enhanced interactionHigh affinityPharmaceutical delivery mechanismImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseAutoimmune Reactions
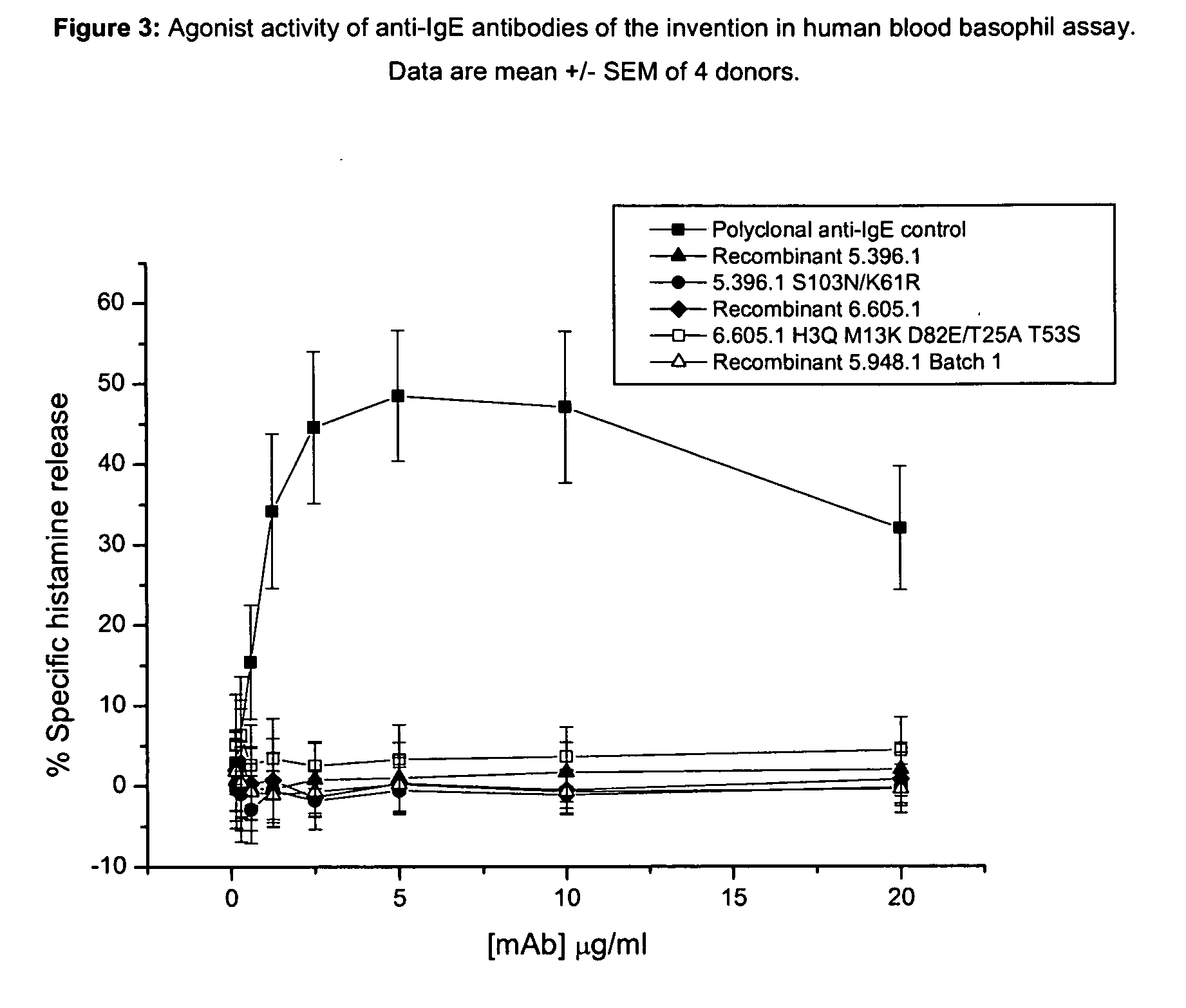
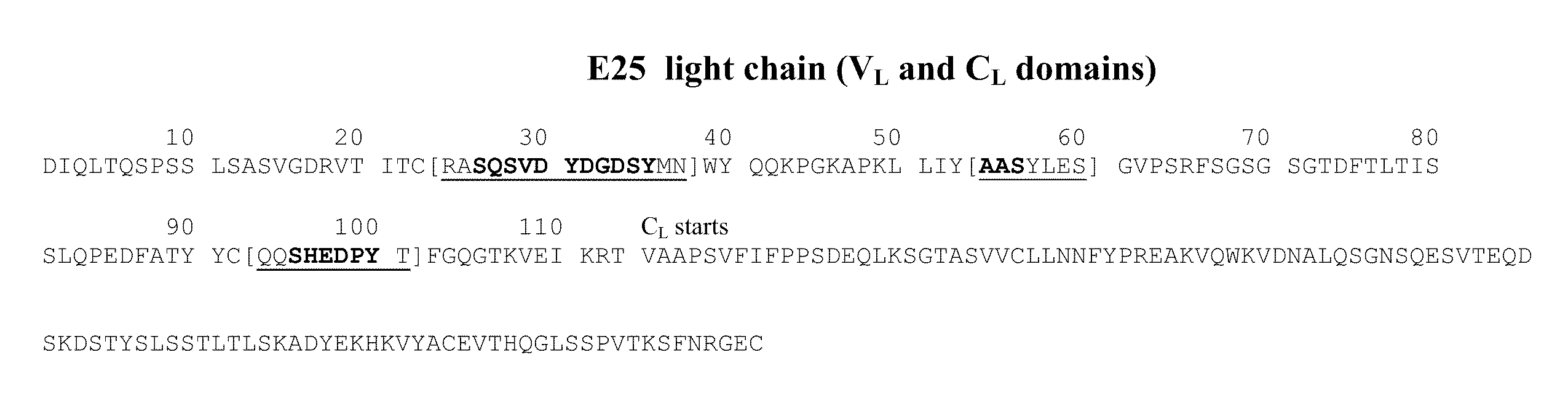
The present invention relates to the area of improved anti-IgE antibodies and antigen binding agents, and compositions thereof, which target IgE, for instance: for use in treating disorders caused by IgE (such as allergic responses, or certain autoimmune responses); and, in particular, disorders caused by the interaction of IgE with the FcεRI receptor. In particular, this invention relates to improved anti-IgE antibodies and antigen binding agents related to novel mutants of omalizumab (Xolair®). The improved anti-IgE antibodies and antigen binding agents of the invention may have improved affinity for IgE and / or an improved interaction with the Cε2 domain of IgE and / or an improved modified epitope on IgE (for instance further involving the Cε2 domain of IgE) and / or the ability to disassociate IgE from the FcεRI receptor for instance at pharmaceutically-relevant concentrations. In one aspect, improved or novel treatments for IgE mediated disorders are disclosed in which IgE is targeted (for instance free IgE and / or IgE complexed with the FcεRI receptor).
Owner:UCB PHARMA SRL
Displaying native human IgE neutralizing FcepsilonRIa-contacting IgE B-cell epitopes by constraining super beta(b)-strands and cystine knots on thermostable protein scaffold
Vaccine displaying native antigenic loops of immunoglobulin E is critical for eliciting neutralizing anti-IgE antibodies. The embodiment of the invention enables the display of native antigenic IgE receptor-contacting loops as IgE B-cell vaccines via three steps of constraining methods. The loops of multiple antigenic B-cell epitopes can be molecularly grafted in, and conformationally constrained by the energy favorable flanking beta (b)-stands, i.e., the super b-strands identified in this invention. The constrained loops can be further stabilized in replacing a selective loop within the cystine knot peptide. These dual constrained antigenic loops are then integrated onto thermostable protein scaffolds, folded in the oxidative milieu that provides further conformational constraint and high yield.
Owner:CHEN SWEY SHEN
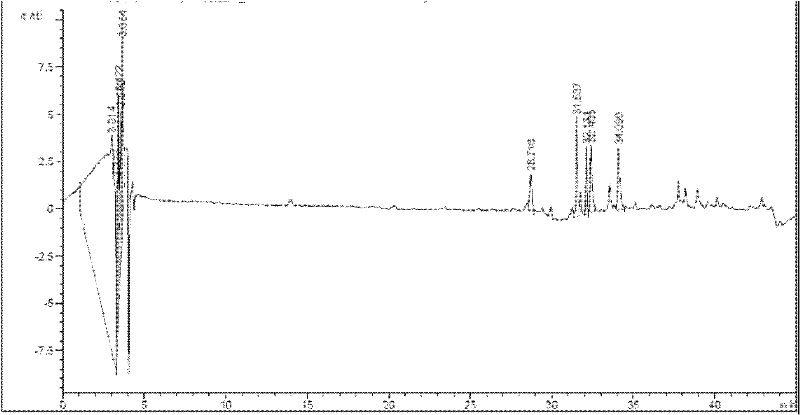
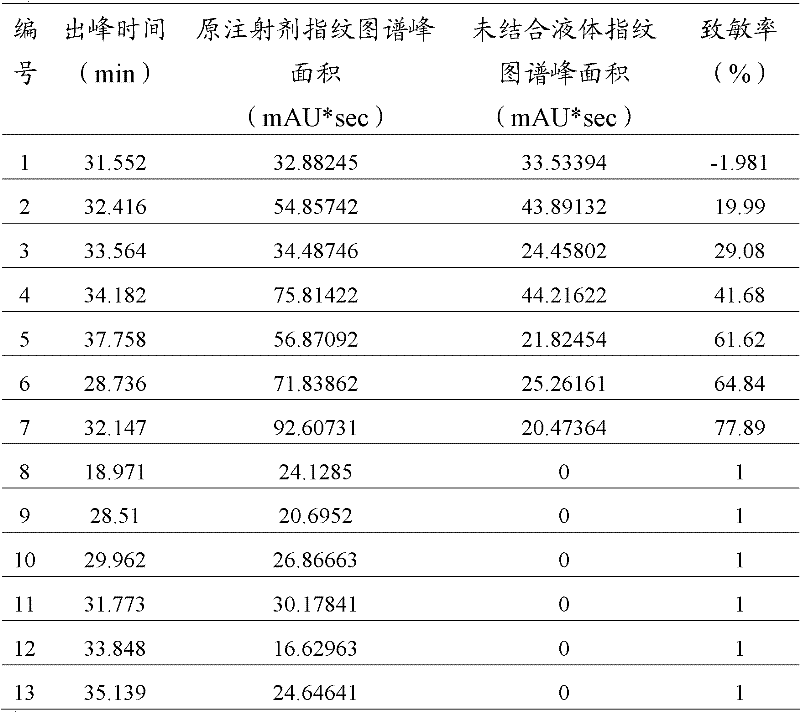
Comprehensive detection method for allergen in traditional Chinese medicine (TCM) injection
InactiveCN102353771AIdentify allergensImprove comprehensivenessMaterial analysisAntigenImmunologic function
The invention discloses a comprehensive detection method for an allergen in a TCM injection, and relates to the field of immunology and science of TCM. According to the invention, the method is based on combined ELISA (enzyme linked immunosorbent assay) principles and fingerprint chromatogram variance analysis, uses an immunoreaction of embedding antigens, IgE antibodies or anti-IgE antibodies with solid carriers and respectively carries out labeled immunoreaction between TCM injection component antigens and labeled anti-IgE antibodies, the embedded IgE antibodies and labeled antigens, the embedded IgE antibodies and known synthetic antigens, the embedded IgE antibodies and enzyme labeled antibodies, the embedded IgE antibodies and IgE antibodies in a blood sample captured by the embedded IgE antibodies, the labeled antigens or antigens and labeled antibodies; the captured IgE antibodies are combined with TCM injection components, and fingerprint chromatogram variance analysis is carried out by using GC / MS and HPLC / MS; specific IgE and corresponding allergens in human and animal blood samples allergic to the TCM injection are determined based on comprehensive consideration of information of a plurality of aspects. The detection method for the TCM injection provided in the invention has the characteristics of high specificity, high sensitivity, high efficiency and easy and convenient operation, is applicable to simultaneous detection of a plurality of components and can effectively prevent omission.
Owner:HUNAN UNIV OF CHINESE MEDICINE
Epsilon immunoglobulin chain derived peptides for induction of anti-IgE antibodies
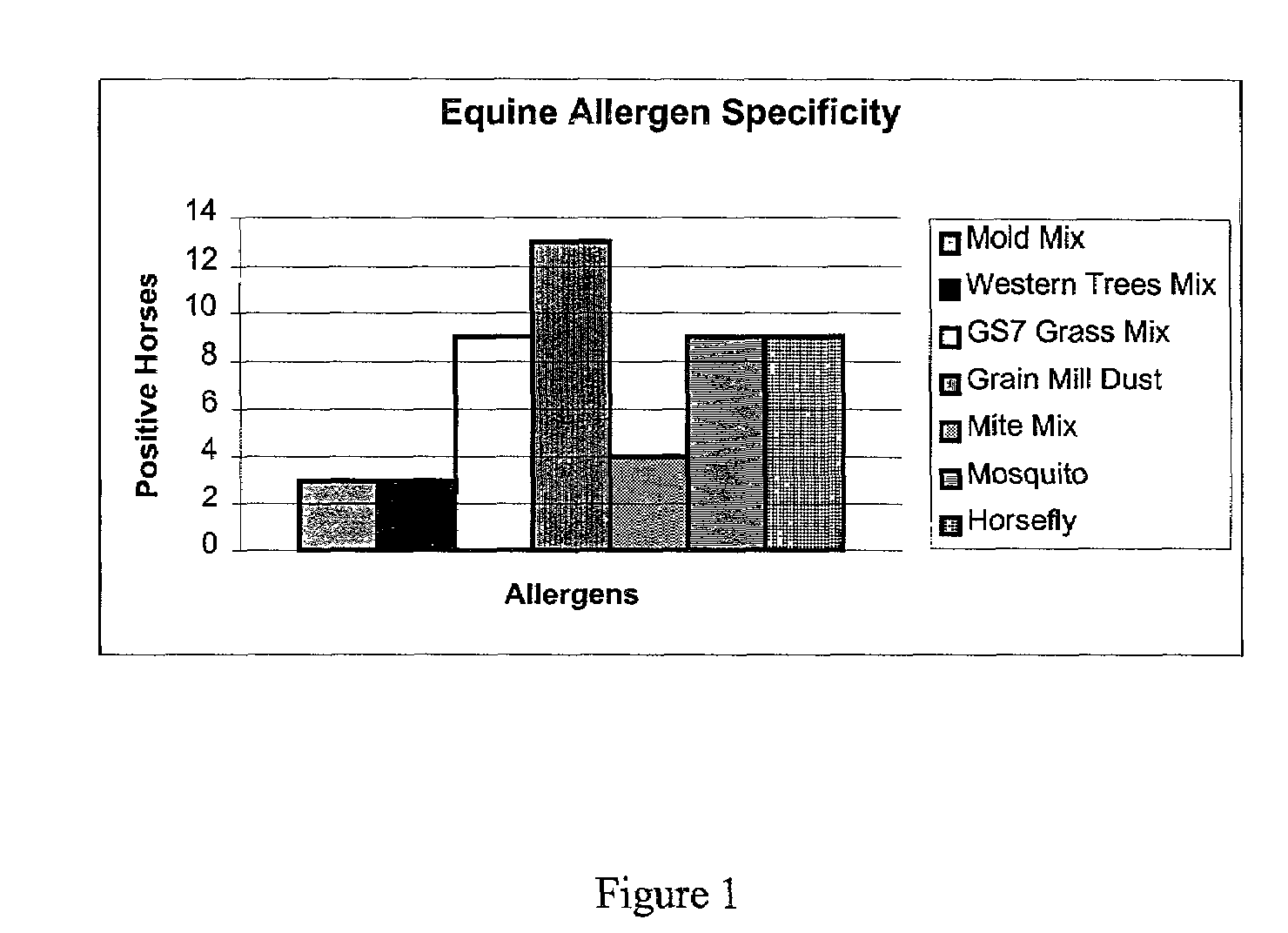
The present invention relates to identification of polypeptides useful for generating antibodies specific for non-human IgE, particularly equine IgE. The invention, therefore, also relates to antibodies that specifically bind to IgE and methods to detect IgE using the antibodies. The invention also provides a kit for detection of IgE.
Owner:RGT UNIV OF CALIFORNIA
Apoptotic anti-ige antibodies
The present application relates to apoptotic anti-IgE antibodies, nucleic acid encoding the same, therapeutic compositions thereof, and their use in the treatment of IgE-mediated disorders.
Owner:GENENTECH INC
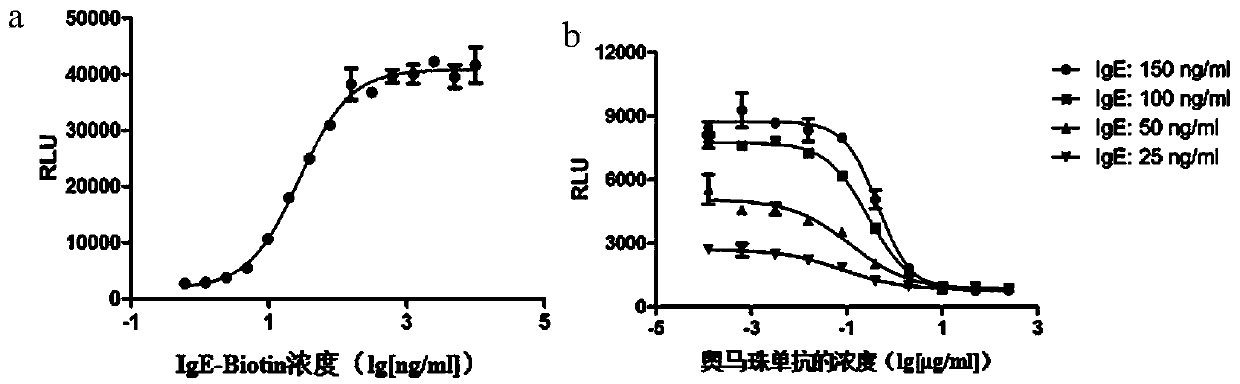
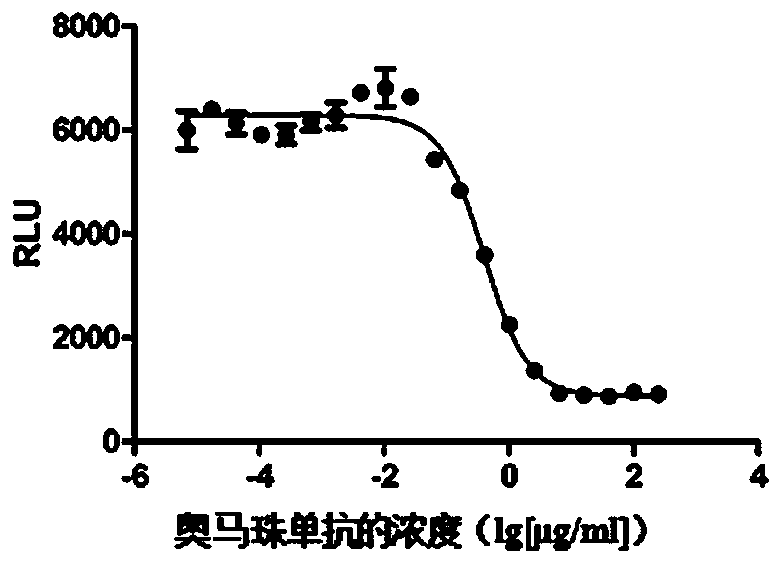
Method for stably measuring biological activity of anti-IgE antibody drug
ActiveCN110358738APromote research and developmentLow costMicroorganism based processesImmunoglobulinsEffector cellCell strain
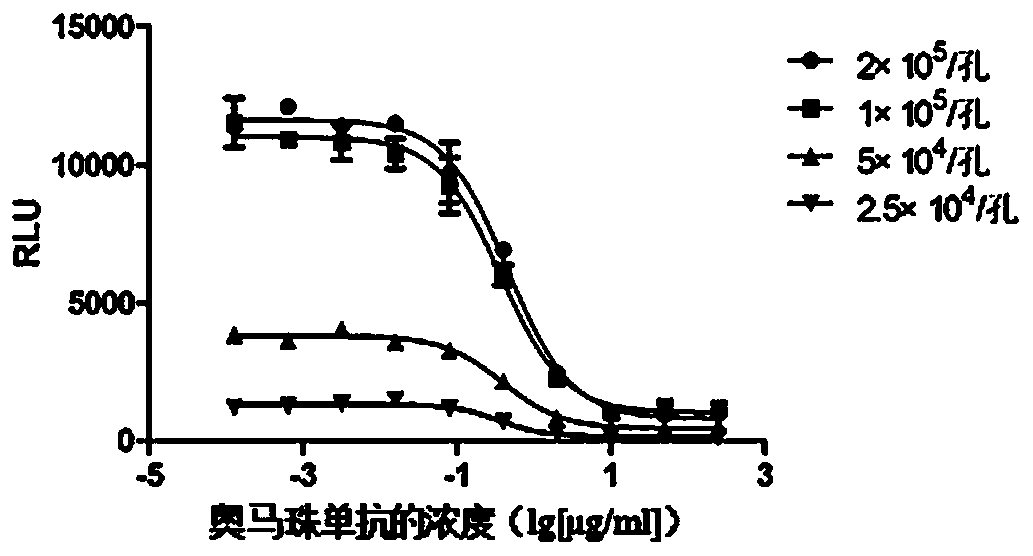
The invention discloses a method for stably measuring biological activity of an anti-IgE antibody drug. The method is based on a biological activity detection method established by constructing an effector cell line stably expressing FcepsilonRIalpha and NFAT reporter genes, can quickly and conveniently detect the biological activity of an anti-IgE antibody, and has higher specificity, sensitivity, accuracy and precision. The invention also discloses a method for constructing a cell line highly expressing FcepsilonRIalpha and containing the reporter genes and a product containing the cell line.
Owner:NAT INST FOR FOOD & DRUG CONTROL +1
Administering IgE antagonists during pregnancy to ameliorate allergic diseases in the offspring
InactiveUS20020006402A1Less immunogenicLow immunogenicityAntibody ingredientsImmunoglobulinsAllergyIge binding
The invention relates to IgE antagonists, including monoclonal antibodies, and their use in ameliorating asthma and allergic diseases in offspring of mothers treated during pregnancy with such antagonists. The preferred IgE antagonists do not induce release of the mediators of allergy. One example of such IgE antagonists are anti-IgE antibodies which bind to secreted IgE, to membrane IgE on the surface of IgE-producing B cells, but not to IgE bound to the Fc∈RI on the surface of basophils or mast cells. Preferably, these antibodies also do not bind to IgE bound to Fc∈RII receptors. It is also preferable if these antibodies have human IgG1 or IgG3 constant regions, as well as further human portions, if desired.
Owner:TANOX
IMMUNOAFFINITY SEPARATION MATERIALS COMPRISING ANTI-IgE ANTIBODY DERIVATIVES
InactiveUS20140124448A1HaemofiltrationSolid sorbent liquid separationSingle-Chain AntibodiesAntibody
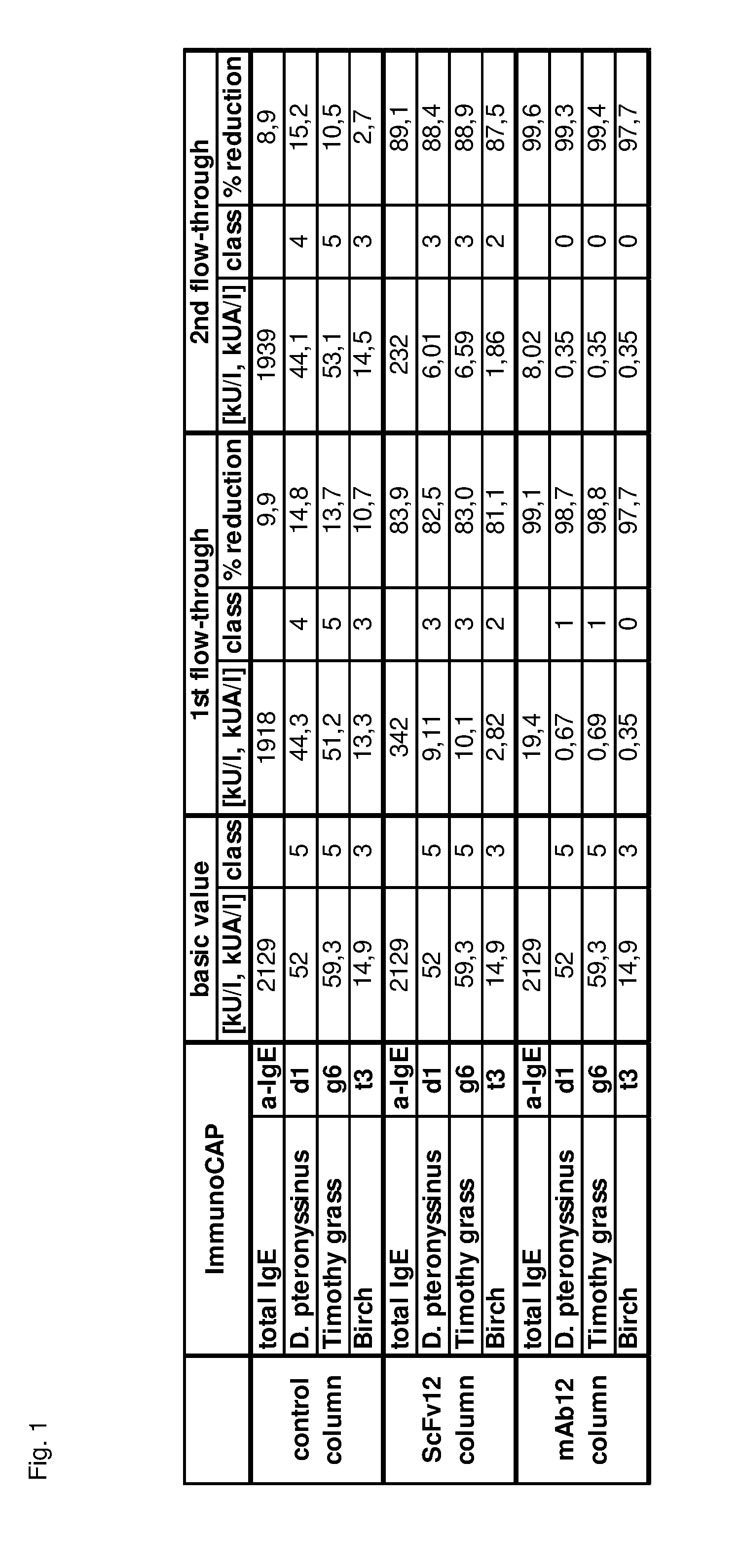
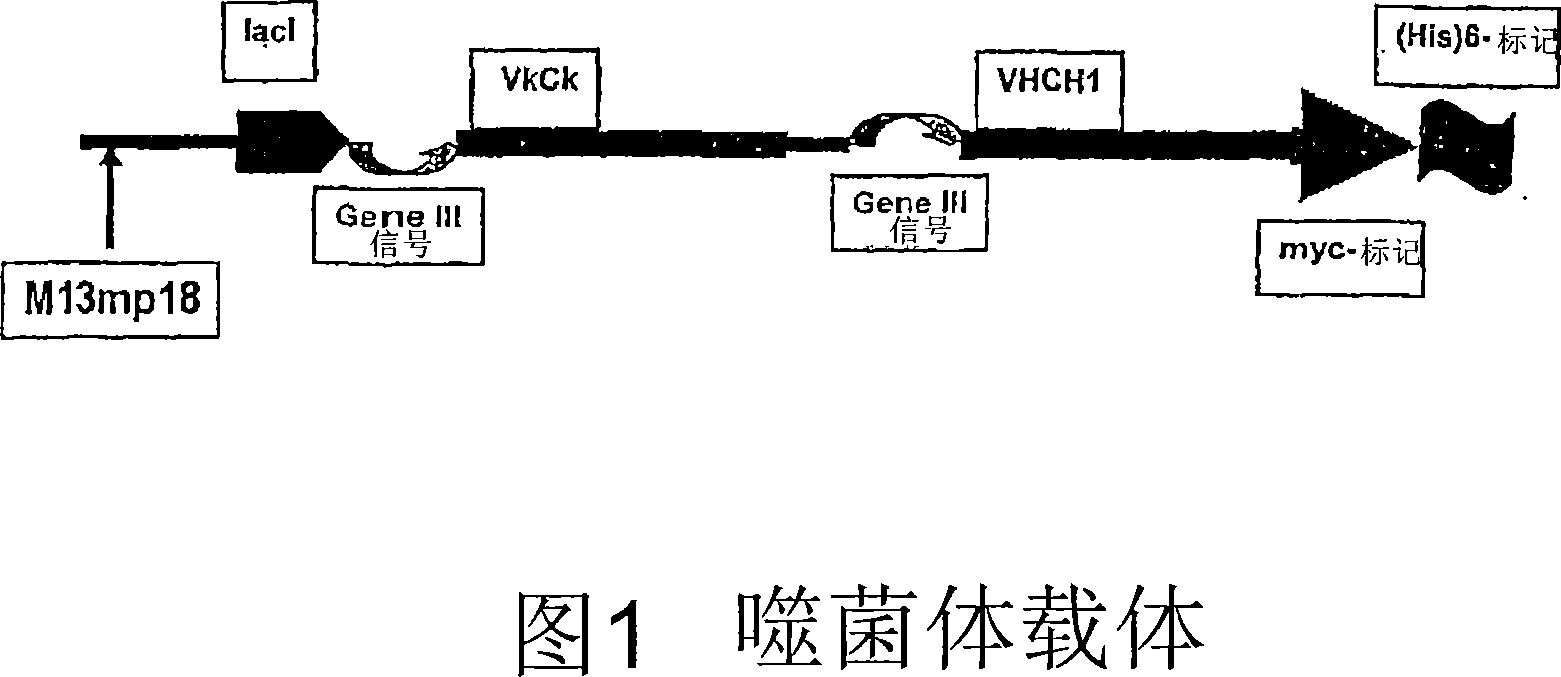
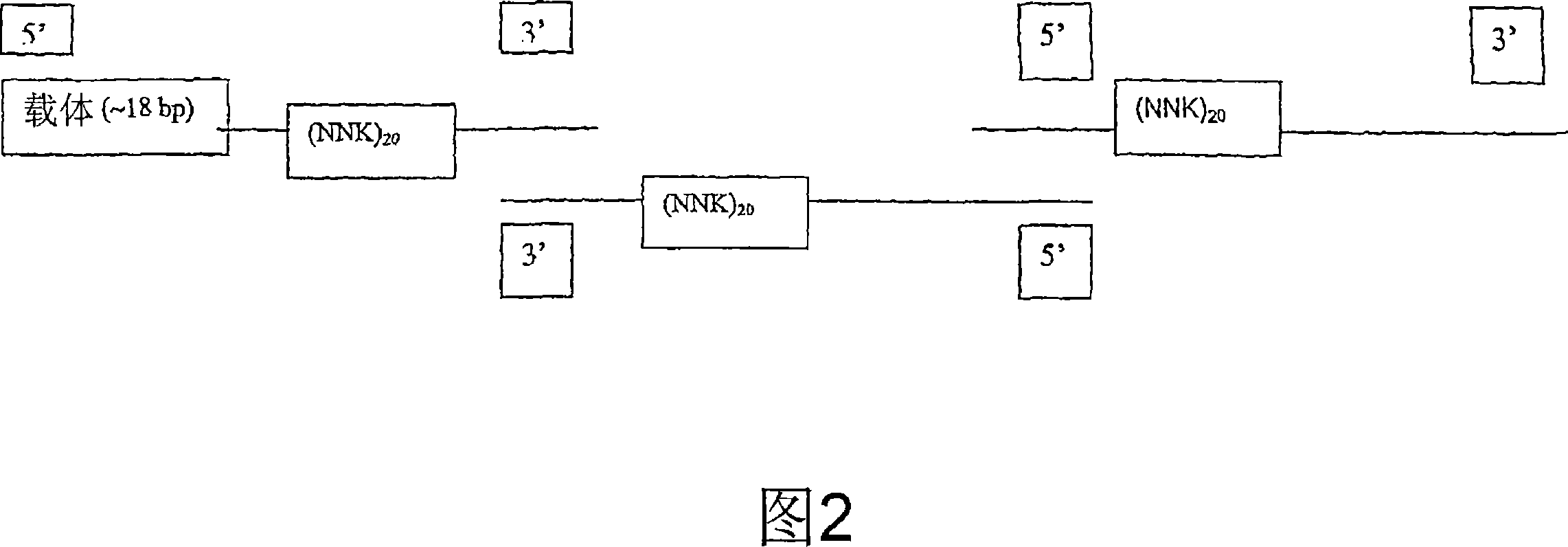
The present invention provides an immunoaffinity separation material, comprising an antibody derivative having high specificity for soluble and cell bound IgE, an apheresis device comprising said material and its use for apheresis, specifically for plasmapheresis. It further provides a recombinant single chain antibody fragment with high specificity for soluble and cell bound IgE that is free of any tag sequences as well as the method for its production.
Owner:BIOMAY AG +1
Human IgE antibody detection kit, and making method and detection method thereof
InactiveCN103175954AHigh detection sensitivityEliminate distractionsMaterial analysisHorseradish peroxidaseHuman ige
The invention discloses a human IgE antibody detection kit. The kit comprises a coated micro-porous plate or its making raw material, a sample diluent, a quality control serum, a concentrated cleaning solution, a substrate solution, a stopping solution, a biotin-labeled anti-IgE antibody binding solution, and a horseradish peroxidase-labeled avidin binding solution. The kit has a high sensitivity and a high specificity because of the adoption of a biotin-avidin amplification system. The invention also discloses a making method of the kit. The labeling efficiency can be improved through the SATA modification of the Fc end of the invariable region of an antibody and the connection of HPDP-LC-biotin and the modified antibody. The invention further discloses a detection method of the human IgE antibody. The human IgE antibody detection kit or the kit made through the making method has the advantages of low cost, simple operation, use convenience, and easy popularization application when the kit is used in detection.
Owner:北京北方北方有限责任公司
Treating atopic dermatitis with lgE antagonists
InactiveCN1289253AFacilitated releaseRelease noPeptide/protein ingredientsHybrid cell preparationMast cellAtopic dermatitis
The invention relates to a pharmaceutical composition for treatment of atopic dermatitis comprising a suitable IgE antagonist which does not induce release of the mediators of allergy; for example, anti-IgE antibodies which bind to secreted IgE, to membrane IgE on the surface of IgE-producing B cells, but not to IgE bound to the Fc epsilon RI on the surface of basophils or mast cells. Preferably, these antibodies also do not bind to IgE bound to Fc epsilon RII receptors. It is also preferable if these antibodies have human IgG1 or IgG3 constant regions, as well as further human portions, if desired. The pharmaceutical composition can be administered systemically or topically.
Owner:泰诺士公司
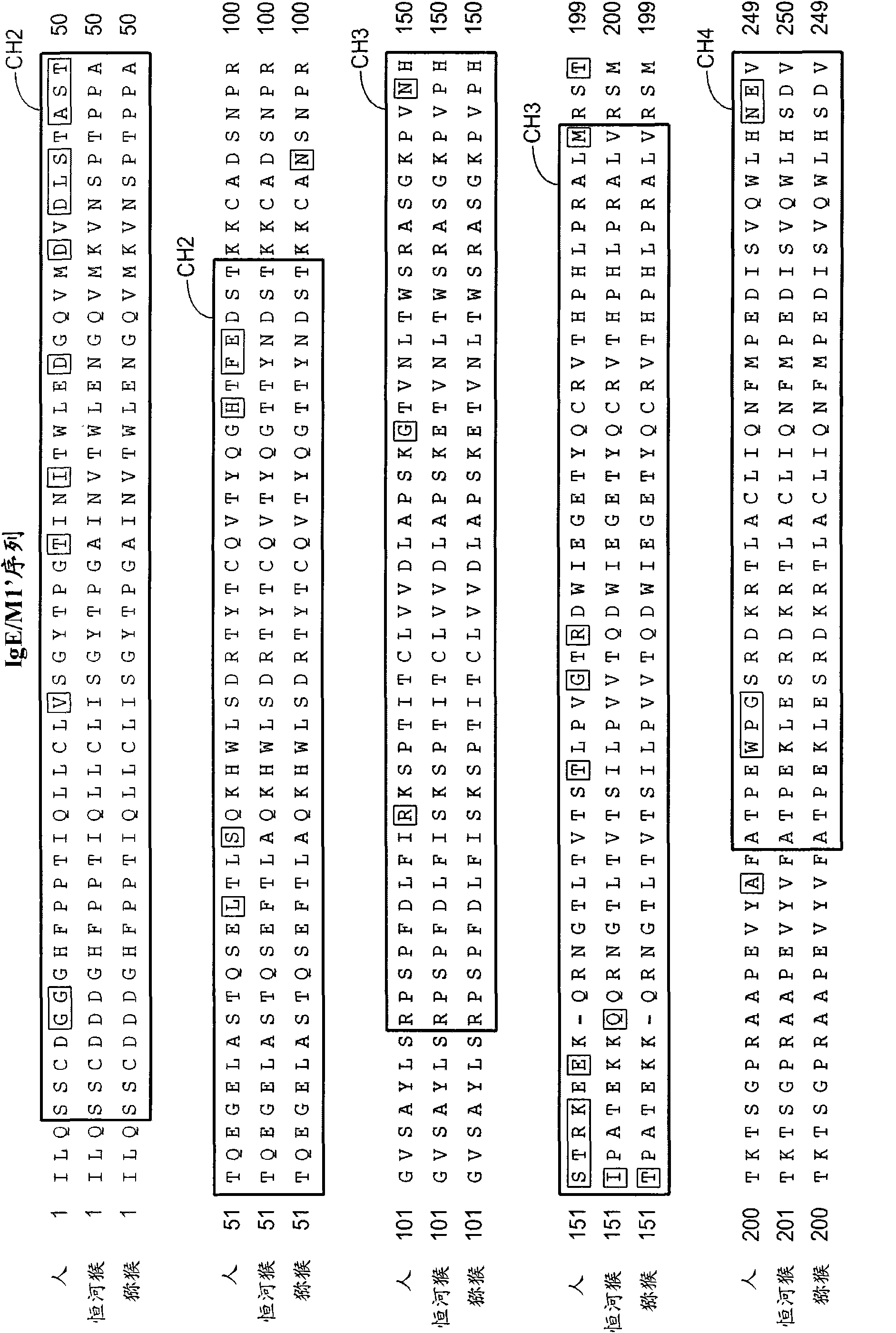
Identification of novel IgE epitopes
InactiveCN1926149AImmunoglobulins against animals/humansAntibody medical ingredientsEpitopeHigh affinity antibody
The present invention relates to novel peptide epitopes derived from the CH3 domain of IgE which are recognized by high affinity antibodies that specifically bind IgE. These novel peptides may be used for both active immunization of a subject by administering these peptides to generate high affinity antibodies in a subject, as well as for generating high affinity anti-IgE antibodies in non-human hosts that specifically bind to these regions of IgE for passive immunization of a subject.
Owner:TANOX
Nucleic acid encoding anti-IgE antibodies
The present invention describes IgE antagonists (including variant anti-IgE antibodies) and their use in diagnosis, therapy or prophylaxis of allergic and other IgE-mediated disorders, including asthma, food allergies, hypersensitivity and anaphylactic reactions.
Owner:GENENTECH INC
IDENTIFICATION OF NOVEL IgE EPITOPES
Owner:TANOX
Method for detecting specific immune globulin E of eriocheir sinensis
ActiveCN107643404ASensitive detectionBiological testingFluorescence/phosphorescenceAntigenSilicon dioxide
The invention discloses a method for detecting specific immune globulin E of eriocheir sinensis and belongs to the crossing field of material and biomedicine. The method includes: adopting fluorescenthollow mesoporous silicon dioxide FHMNs as a carrier resistant to IgE antibody and Fe3O4@SiO2 prepared by coating silicon dioxide with ferroferric oxide as an allergen carrier; allowing the two materials to capture a target-the specific IgE of eriocheir sinensis at the same time; utilizing antigen-antibody specific reaction to gather and separate the target-sIgE; utilizing fluorescent molecular signals in FHMNs to amplify signals of low-concentration target sIgE; correlating fluorescent intensity with IgE concentration to quickly and sensitively detect specific IgE of eriocheir sinensis in human blood. By the method, IgE detection limit is lowered effectively, and detection time is shortened.
Owner:JIANGNAN UNIV
High-throughput in-vitro allergen screening method
InactiveCN105510578AEfficient detectionImprove throughputMaterial analysisBiotin-streptavidin complexScreening method
The invention discloses a high-throughput in-vitro allergen screening method, which comprises: peripheral blood drawing, separating serum, adding the serum into BSA liquid closed 96-well plates pre-coated with mouse or rabbit anti-human IgE antibody in advance to perform incubation; carrying out specific antigen-antibody reaction; after incubation, conducting washing to remove non-specifically bound components; adding 96 biotin labeled specific allergens to one of the 96-well plates respectively; adding a biotin labeled anti-IgE antibody into the other 96-well plate, performing incubation, conducting washing to remove non-specifically bound components; conducting sample adding of fluorescein labeled streptavidin to the 96-well plates, conducting inoculation, and then performing washing; then sending the 96-well plates to a high-throughput fluorescence signal detection system to carry out quantitative detection of the fluorescence intensity respectively, and using the total IgE detected by the first 96-well plate to judge allergy; and using the other 96-well plate to diagnose allergen. The method provided by the invention has an accuracy of effective detection of allergens up to 95%, and can detect more than 90 allergens at a time.
Owner:SUZHOU AILEZHEN MEDICAL EQUIP CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com