Apoptotic anti-ige antibodies
An antibody and apoptosis technology, applied in the direction of antibodies, drug combinations, anti-infective drugs, etc., can solve problems such as B cell apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
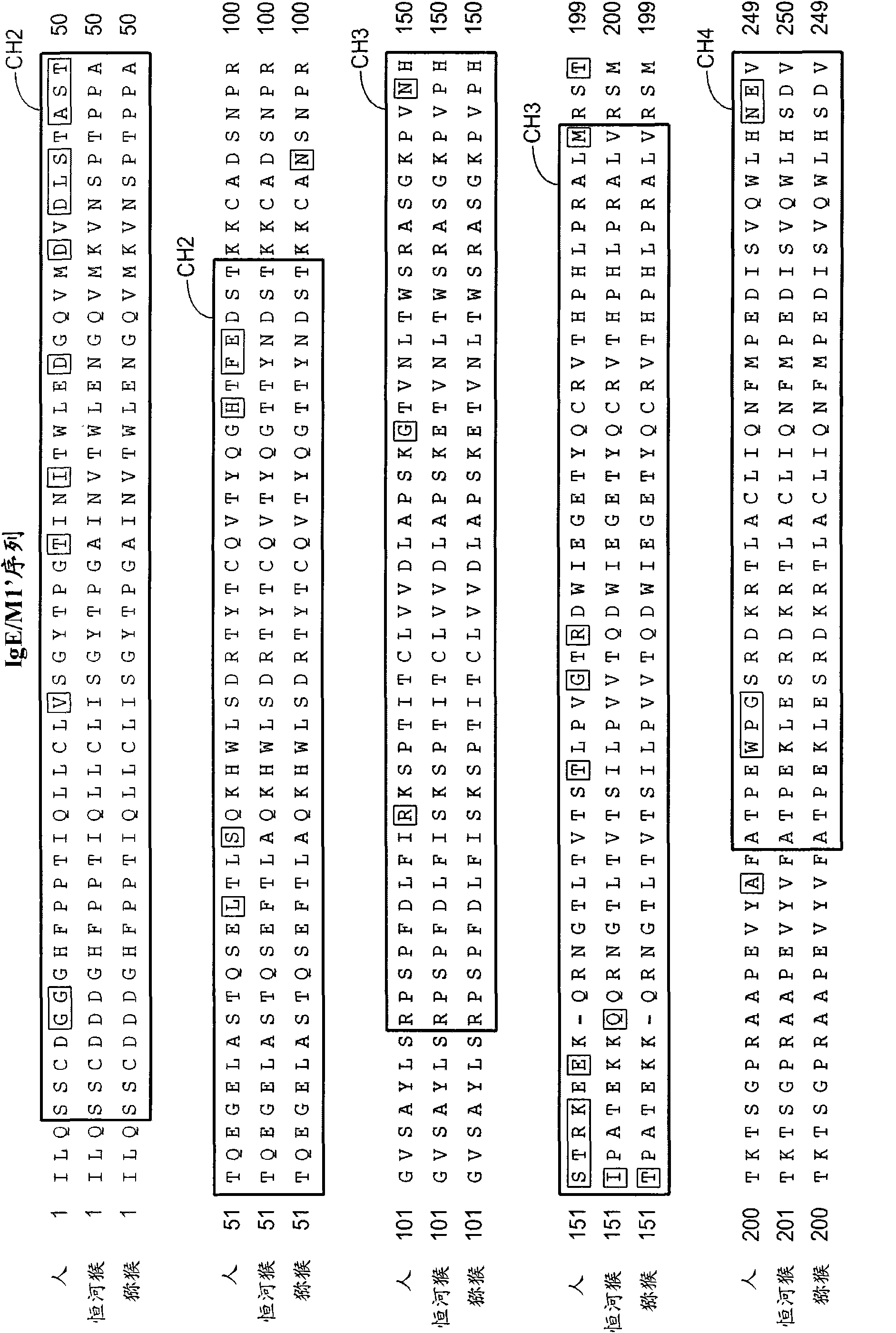
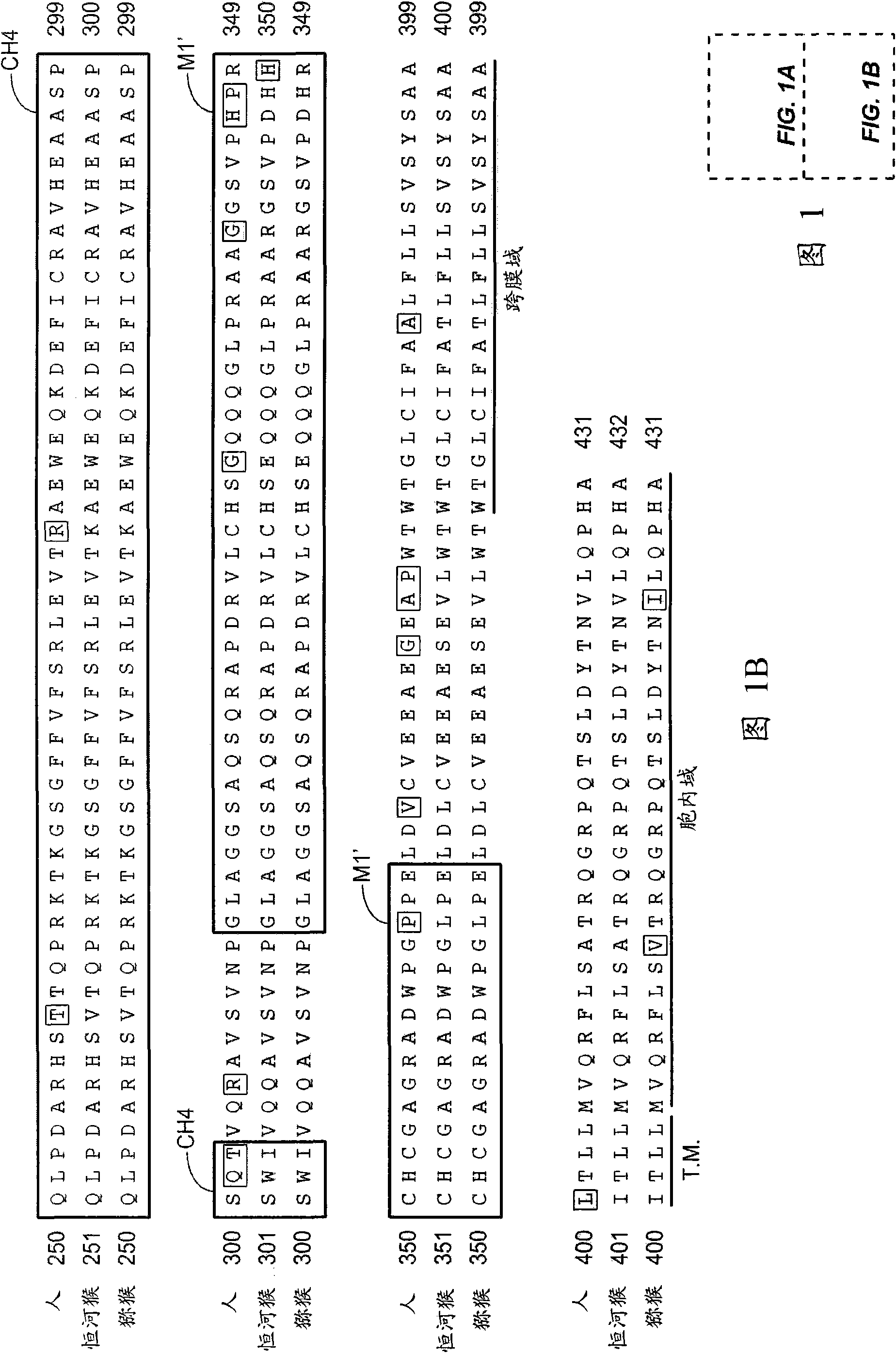
[0422] Example 1: Isolation of primate IgE
[0423] Human IgE was cloned from the human IgE myeloma cell line U266 (ATCC, Manassas, VA, TIB #196). Rhesus and macaque IgE sequences were determined by cloning and sequencing of IgE from cDNA derived from rhesus and macaque peripheral blood mononuclear cells (PBMCs) that had been stimulated to generate IgE-switched cells (PBMCs). Forward and reverse primers (shown below) were designed from human IgE sequences. The upstream forward primer was designed just upstream of the CH2 domain. The reverse primer was designed at the end of the transmembrane domain. Rhesus and macaque PBMCs were obtained from California National Primate Research Center, Davis, CA. Will 5x10 6 Each PBMC was cultured with IL-4 (100 ng / ml) and CD40L (3 μg / ml) (R&D Systems, Minneapolis, MN, #204-IL and #617-CL, respectively) for 4 days. Cells were then harvested, RNA was prepared (RNeasy Mini Kit, #74106, Qiagen, Valencia, CA), and cDNA was prepared using the...
Embodiment 2
[0429] Example 2: Isolation of Anti-IgE / M1' Antibody
[0430] Generation of CH3-CH4-M1' / Fc fusion protein
[0431] The human IgE CH3-CH4-M1' / Fc fusion protein was generated by PCR amplifying the human IgE CH3-CH4-M1' domain from cDNA prepared from U266 cells (ATTC Manassas, VA, TIB #196), plus An additional 15 amino acids just downstream of the M1' domain. RNA was prepared from U266 cells (RNeasyMini Kit, #74106, Qiagen, Valencia, CA) and cDNA was prepared using the BD SprintPowerscript Oligo dT Priming Kit (BD Biosciences, San Jose, CA, #639558). RT-PCR was performed and PCR products were cloned by TOPO-TA cloning (Invitrogen, Carlsbad, CA, #45-0641). The N-terminal signal sequence was added by PCR mutagenesis for expression in mammalian cells, and the signal sequence +CH3+CH4+M1'+15 amino acids were cloned into an expression vector (pRK huIgG1 Fc; Genentech) to generate C-terminal fusion of IgG1 Fc region. The sequence of the final protein (including signal sequence and ...
Embodiment 2A
[0568] Example 2A: Humanization of Anti-IgE / M1' Antibodies
[0569] This example describes the humanization of murine anti-IgE / M1' antibodies 26A11, 7A6 and 47H4.
[0570] Materials and methods
[0571] The M1' domain of membrane-bound human IgE was expressed as an Fc fusion in CHO cells and purified by conventional means. Hybridomas expressing antibodies 26A11, 7A6 and 47H4 were obtained by immunizing mice with recombinant M1'-Fc fusion protein and identified by ELISA using M1'-Fc-coated plates. Functional antibodies are identified by their ability to bind M1'-expressing cells and promote apoptosis.
[0572] Cloning of murine 26A11, 7A6 and 47H4 variable domains - Total RNA was extracted from hybridoma cells producing 26A11, 7A6 or 47H4 using standard methods. The light chain variable domain (VL) and heavy chain variable domain (VH) were amplified using RT-PCR with degenerate primers for the heavy and light chains. The forward primers are specific for the N-terminal ami...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com