Method for detecting specific immune globulin E of eriocheir sinensis
A Chinese mitten crab and immunoglobulin technology, which is applied in the cross-field of materials and biomedicine, can solve the problems of unstandardized sensitization efficiency, interference of allergy test results, high detection limit, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Preparation of fluorescent probes with different particle sizes FHMNs@PDDA@PAA
[0028] Dissolve cetyltrimethylammonium bromide (CTAB) powder in a mixture of 29mL deionized water, 12mL ethanol and 1mL ammonia water, then add polystyrene microsphere dispersion (dispersed in 10ml deionized ionized water), after magnetically stirring at room temperature for 60 min, tetraethyl orthosilicate (TEOS) was added dropwise, maintaining a constant stirring speed of 150 rpm / min, and continuing to stir at room temperature for 48 h. After the reaction was completed, the reaction solution was vacuum filtered and washed several times, and then dried in a vacuum oven at 65°C for 12 hours to obtain core-shell PS / SiO 2 Composite microsphere powder. Calcined at 550°C for 8h to remove template polystyrene to obtain hollow silica. Disperse 0.05g of HMNs and 0.012g of 5(6)-fluorescein isothiocyanate (FITC) in 5ml of deionized water, stir at room temperature for 48h, centrifuge the...
Embodiment 2
[0030] Example 2: Magnetic probe Fe 3 o 4 @SiO 2 Preparation of @PAA Nanoparticles
[0031] Weigh 300mgFe 3 o 4 Disperse in a mixture of 40mL ethanol and 4mL deionized water, after ultrasonication for 15min, maintain a certain stirring speed, add 5mL ammonia water, slowly add 2mLTEOS, react at room temperature for 12h, and use 0.1moL / L hydrochloric acid solution and deionized water after magnetic separation Wash each for 3 times, and dry at 40° C. for 12 hours to obtain a reddish-brown precipitate, which is ferric oxide / silicon dioxide core-shell nanoparticles. Fe will be produced 3 o 4 @SiO 2 Dispersed in dimethylamide (DMF), mixed with 33ml DMF dissolved in 2g polyacrylic acid (PAA), ultrasonicated for 30min, the mixture was vigorously stirred and heated to 110°C, added 3.3mL DMF dissolved in 4-dimethylaminopyridine and Dissolve N,N'-dicyclohexylcarbodiimide (DCC) in 6.6mL DMF, keep the mixture at 110°C and stir for 12h, then magnetically separate the product, wash w...
Embodiment 3
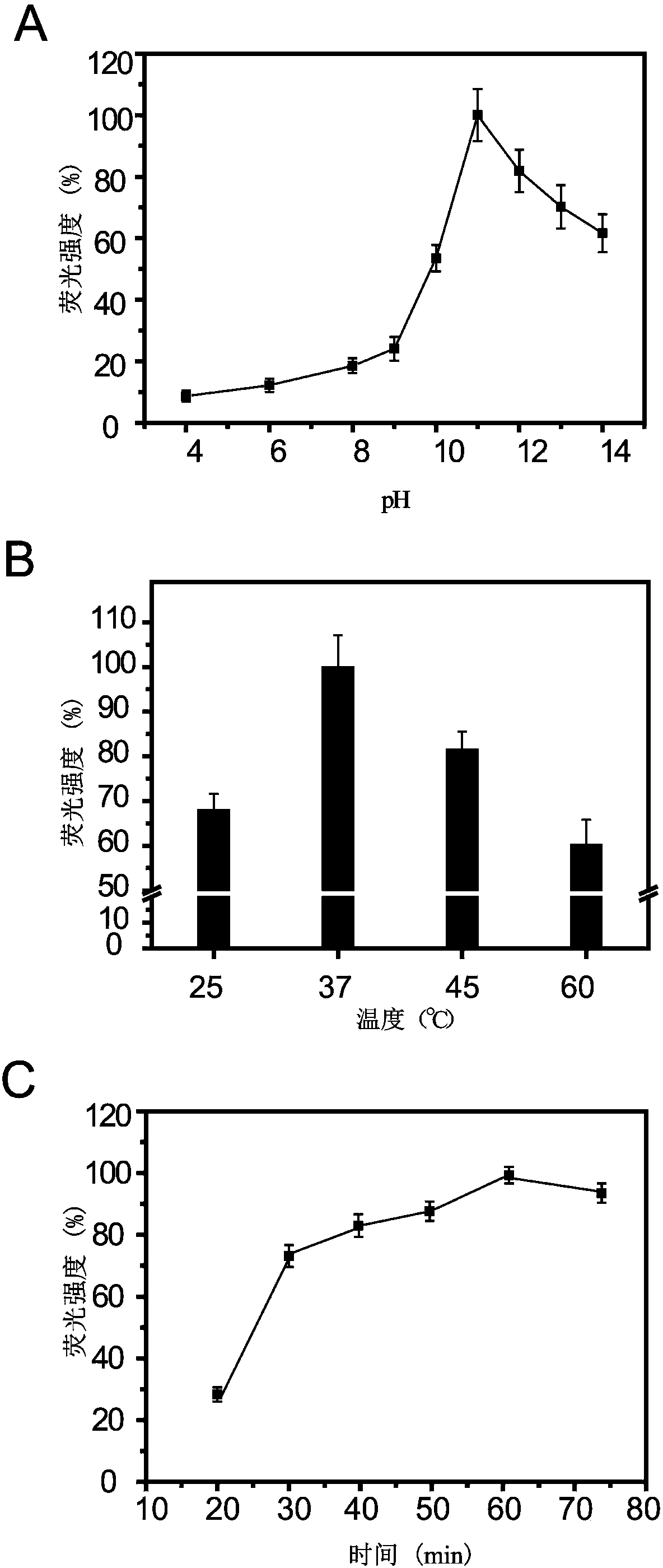
[0033] Embodiment 3: the establishment of allergy detection system fluorescent sensing platform (EFSP)
[0034] (1) Take 50 mg of prepared FHMNs@PDDA@PAA and activate PAA with 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (EDC) / N-hydroxysuccinimide (NHS) In the carboxyl group, take an appropriate amount of activated FHMNs@PDDA@PAA and add a certain concentration of anti-IgE antibody (Ab 2 ), incubated at 37°C for 2h, centrifuged to remove the supernatant, washed 3 times with PBS, added blocking solution at 37°C for 1h, centrifuged to remove the supernatant, and collected the prepared FHMNs@PDDA@PAA-(Ab 2 ) were dispersed in PBS buffer and stored at 4°C for later use.
[0035] (2) the prepared Fe 3 o 4 @SiO 2 @PAA nanoparticles, the carboxyl group in PAA was activated by EDC / NHS, the purified hemocyanin, the main allergen of Chinese mitten crab, was added, incubated at 37°C for 120min, the supernatant was discarded by magnetic separation, washed 3 times with PBS buffer, added...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com