Method for stably measuring biological activity of anti-IgE antibody drug
A biological activity and antibody drug technology, applied in the direction of microorganism-based methods, biochemical equipment and methods, chemical instruments and methods, etc., can solve problems such as poor stability, large variation, and inability to sensitively indicate changes in the activity of omalizumab , to achieve low cost, high accuracy, stable and reliable results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 Screening of RBL-2H3 cell lines stably expressing FcεRIα and NFAT-luc
[0051] 1. Experimental materials
[0052] RBL-2H3 cells were derived from ATCC; pcDNA3.1[FcεRIα / G418] plasmid was purchased from Jinweizhi Biotechnology Co., Ltd.; pGL4.30[luc2P / NFAT-RE / Puro] plasmid was purchased from Jinweizhi Biotechnology Co., Ltd.; experimental use IgE-Biotin (IgE-Biton) was provided by Shanghai Taiyin Biotechnology Co., Ltd.; Bright-Glo luciferase kit was purchased from Promega.
[0053] 2. pcDNA3.1[FcεRIα / G418] plasmid transfection
[0054] RBL-2H3 cells were transfected with pcDNA3.1[FcεRIα / G418] plasmid using Lipofectamine 3000 transfection reagent (Invitrogen). After 24 hours of transfection, 1 mg / mL G418 was added for pressurized screening. After the cell density and viability were restored, the limited dilution method was used to screen monoclonals on a 96-well plate at a density of 1 cell / well. During cell growth, observe and mark which wells are monoclona...
Embodiment 2
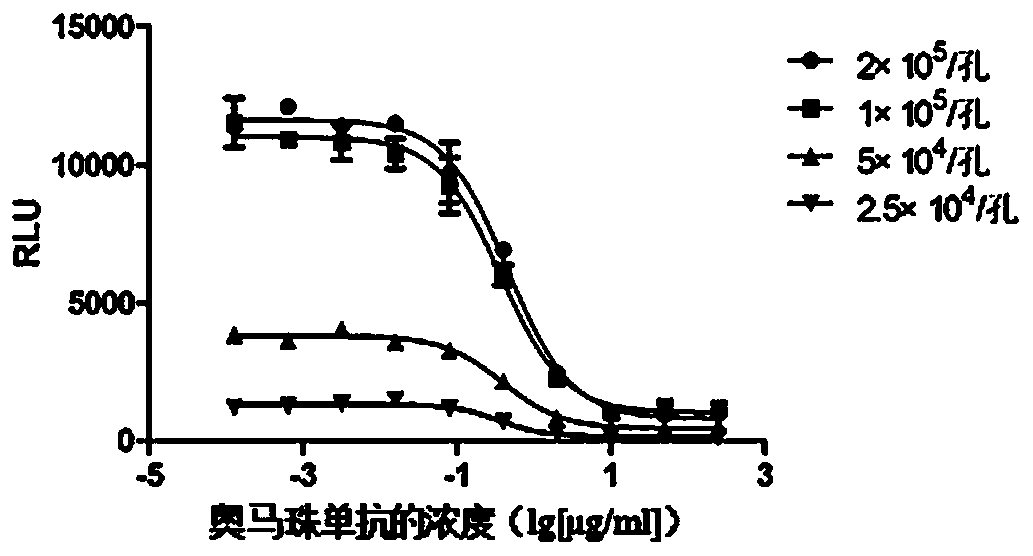
[0066] Example 2 Detection method optimization
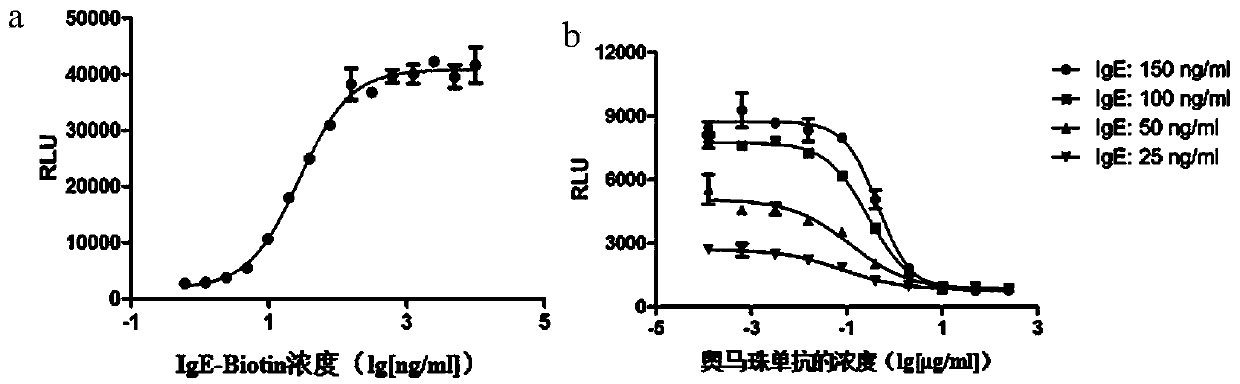
[0067] 1. IgE-Biotin concentration optimization
[0068] IgE-Biotin can bind to RBL-2H3 / FcεRIα / NFAT-luc cells, and after cross-linking with streptavidin, it can stimulate cell degranulation and NFAT-luc reporter gene expression, and anti-IgE antibody can inhibit the occurrence of this process . Therefore, it is necessary to select an appropriate concentration of IgE-Biotin, so that the cells can produce sufficient degranulation reaction after being stimulated, and the reagents will not be wasted due to the concentration reaching the plateau.
[0069] Method: RBL-2H3 / FcεRIα / NFAT-luc cells were divided into 1×10 5 Add cells / well to 96-well white plate, IgE-Biotin concentration starts at 10000ng / mL, dilute 15 concentration points at 1:2 ratio, add to the above cell plate, 37°C, 5% CO 2 Incubate overnight in the incubator. Discard the liquid in the plate the next day, add 20 μg / mL streptavidin 100 μL / well, 37 ° C, 5% CO 2 In th...
Embodiment 3
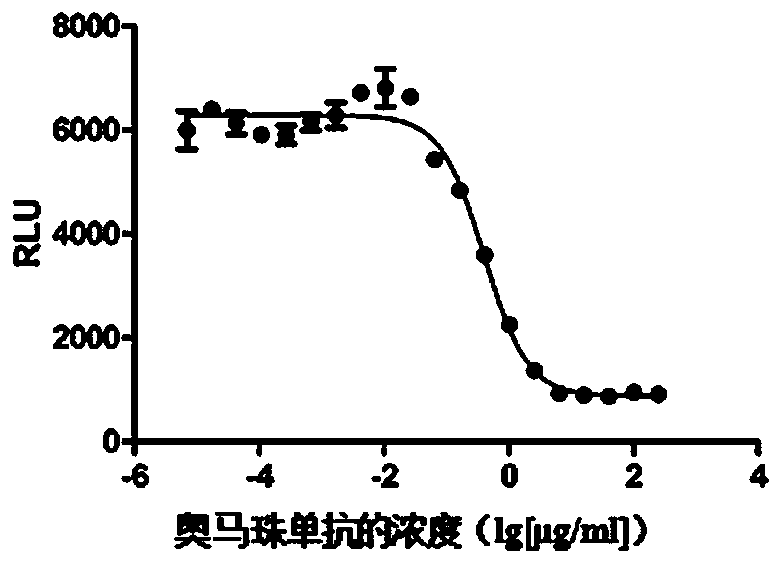
[0088] Verification of embodiment 3 detection method
[0089] 1. Exclusive verification
[0090] This method is aimed at the biological activity of anti-IgE antibodies. Therefore, monoclonal antibody drugs with different targets were used to verify its specificity: omalizumab (Omalizumab, whose target is IgE), rituximab ( Rituximab (targets CD20), Pertuzumab (targets HER2) and Tocilizumab (targets IL-6R). Under the same experimental conditions, the activity of the four monoclonal antibodies was determined using the RBL-2H3 / FcεRIα / NFAT-luc reporter gene assay system.
[0091] Method: RBL-2H3 / FcεRIα / NFAT-luc cells were divided into 1×10 5 Each / well was added to a 96-well white plate; the final concentration of IgE-Biotin was 100ng / mL, the four kinds of monoclonal antibodies were diluted to the initial concentration of 250μg / mL, and 10 concentration gradients were diluted 1:4, mixed with IgE-Biotin and transferred to the above In cell plate; 37°C, 5% CO 2 Cultivate for 15-21h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com