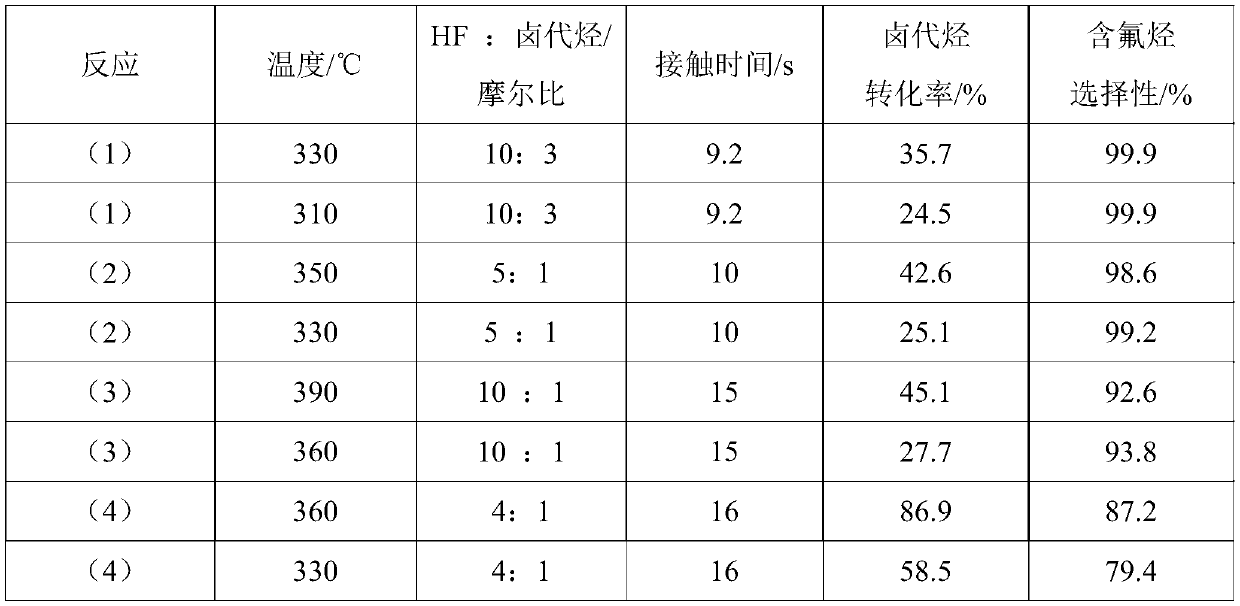
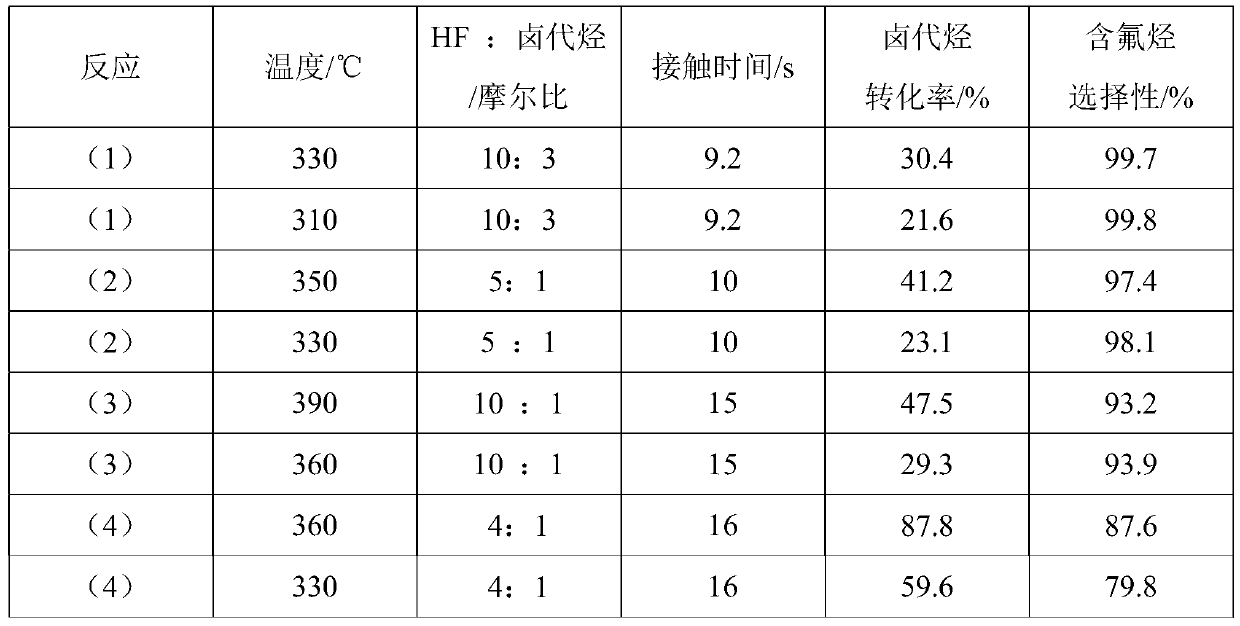
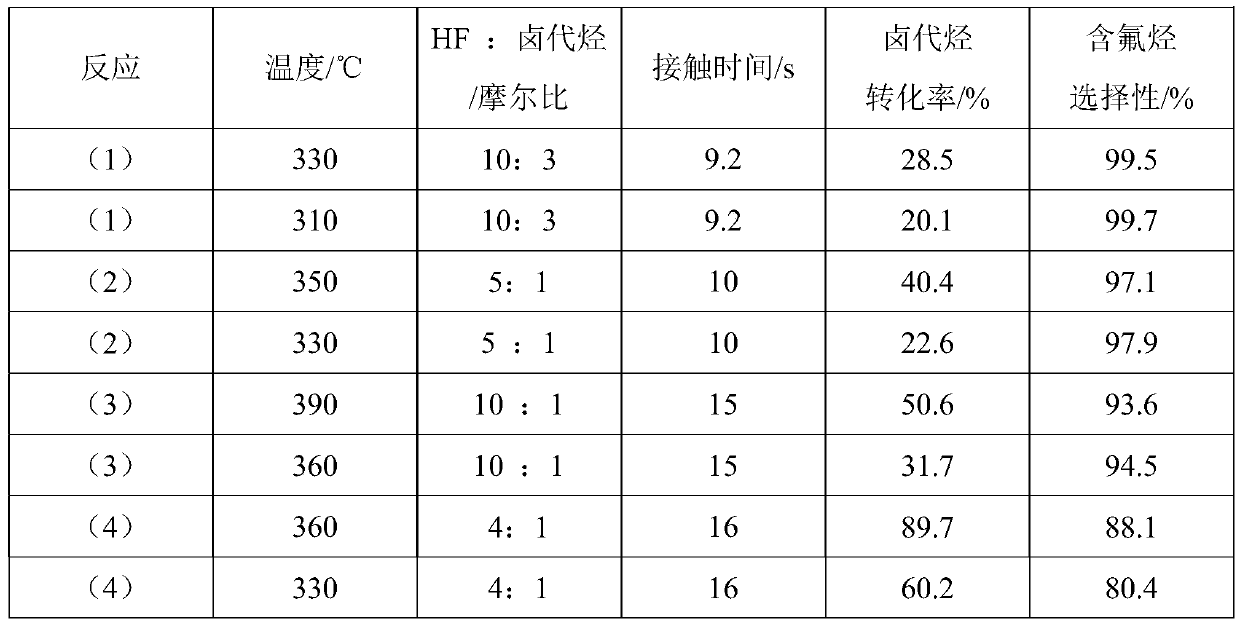
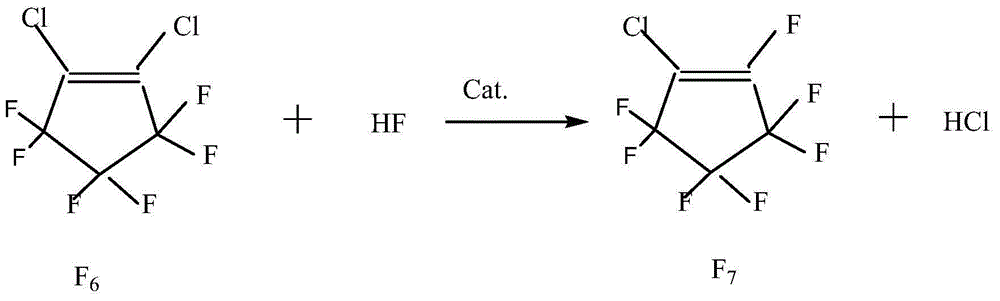
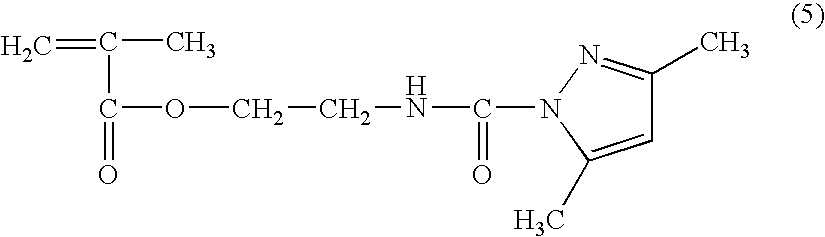
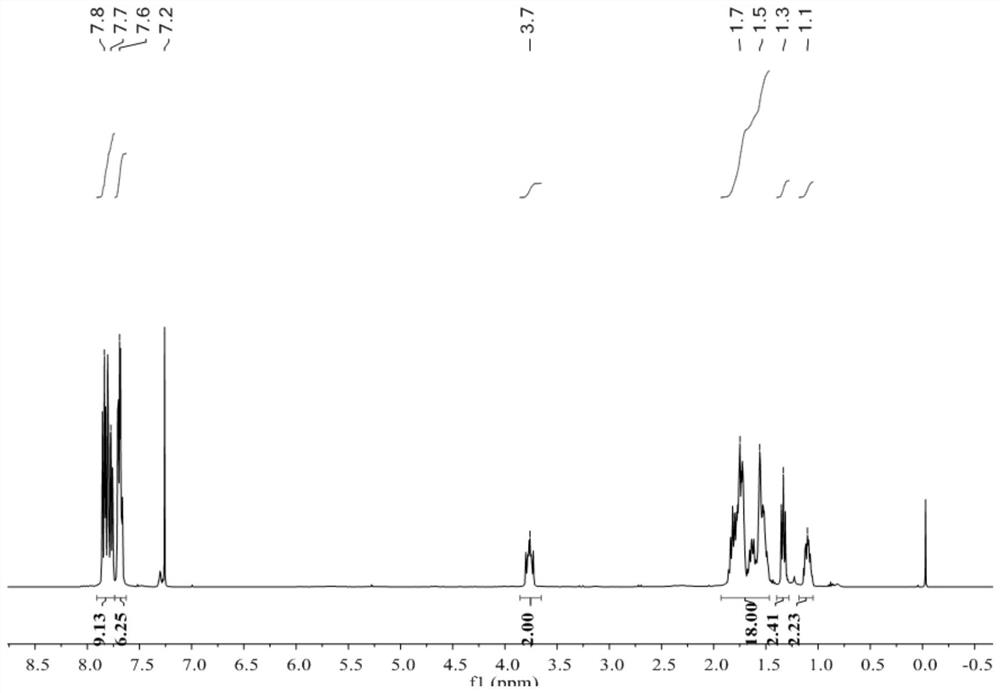
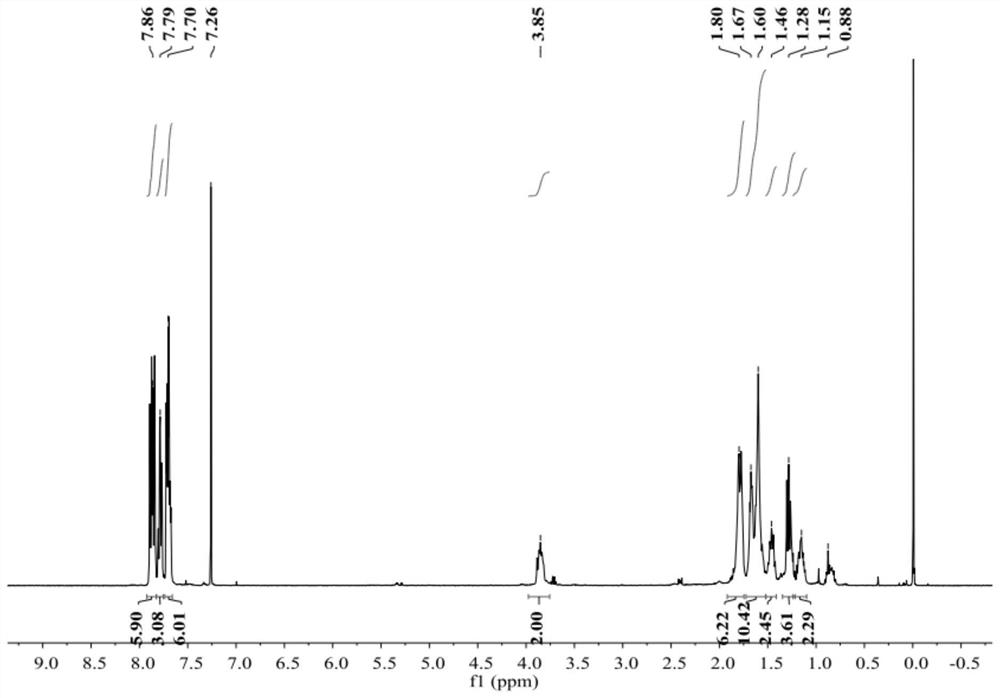
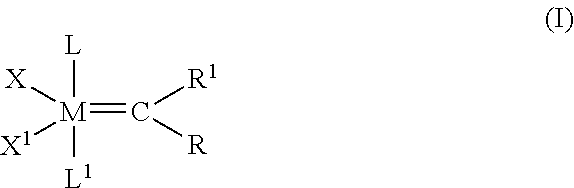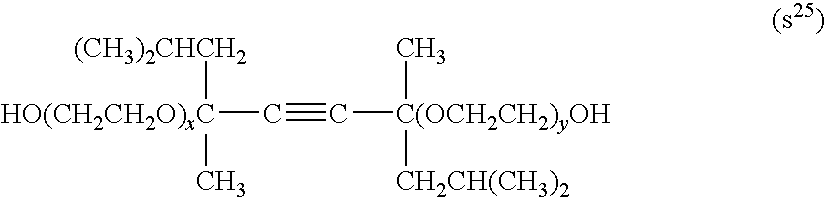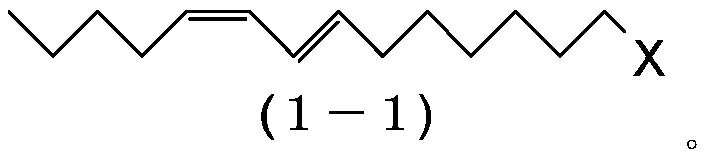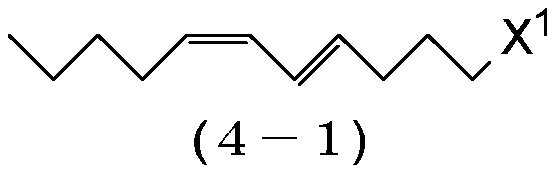Patents
Literature
92 results about "Haloalkene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
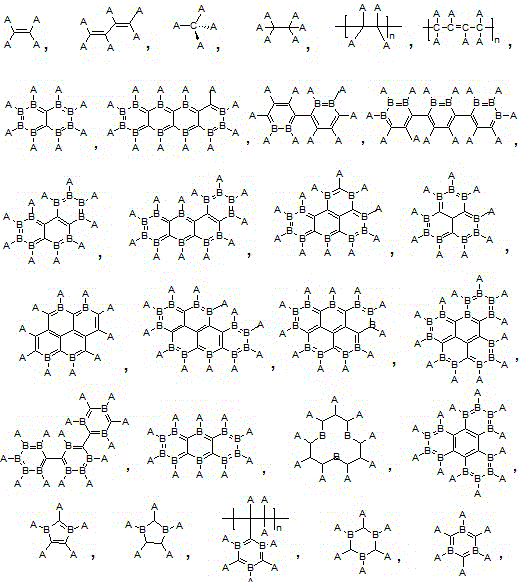
Halogenated unsaturated aliphatic hydrocarbon.
Luminescent element material and luminescent element comprising the same
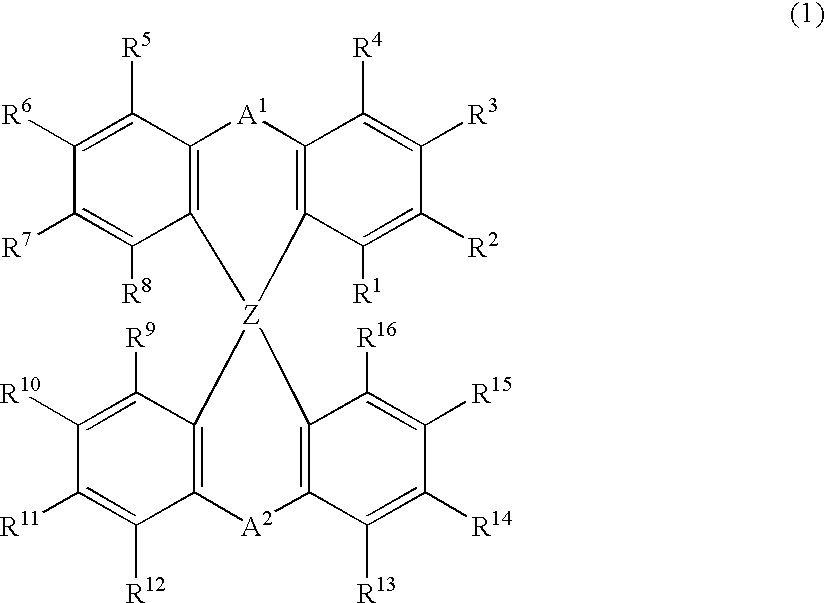
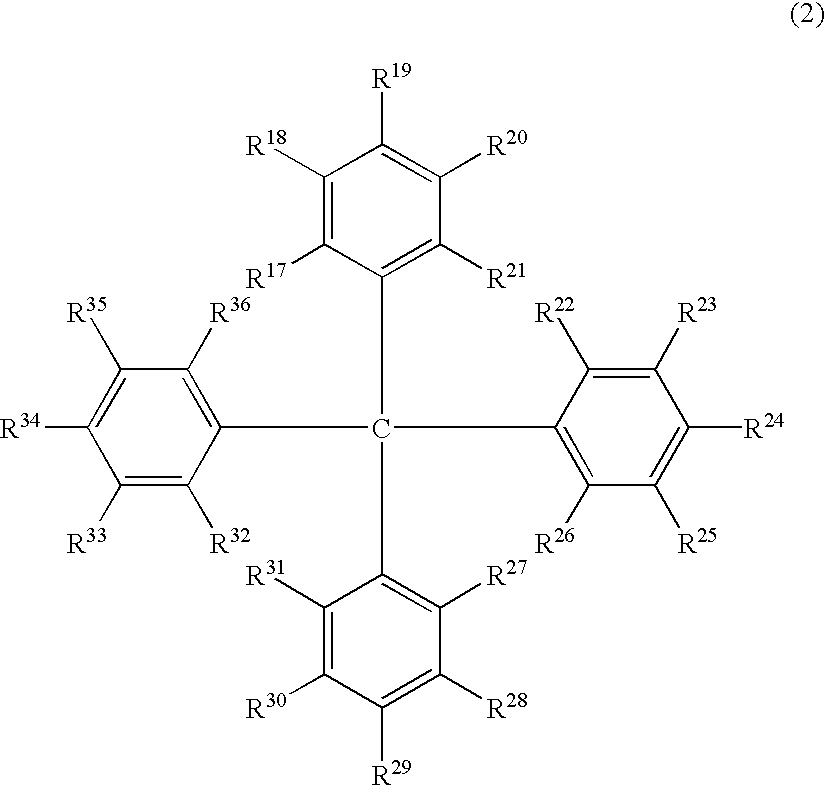
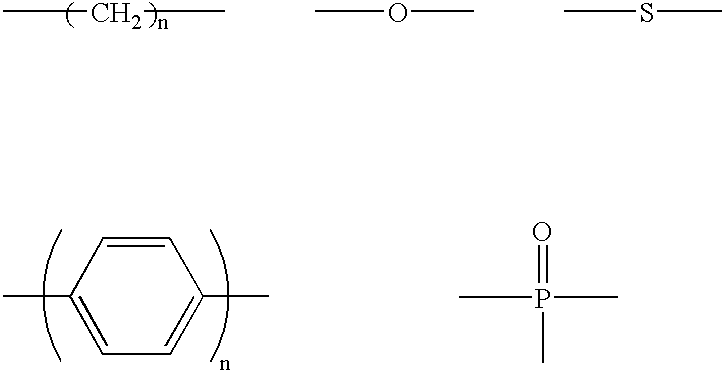
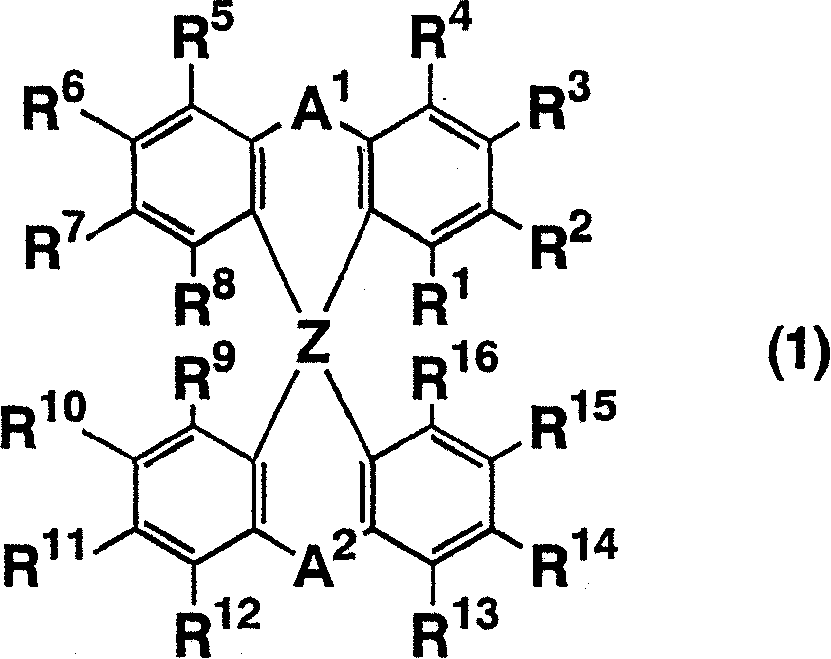
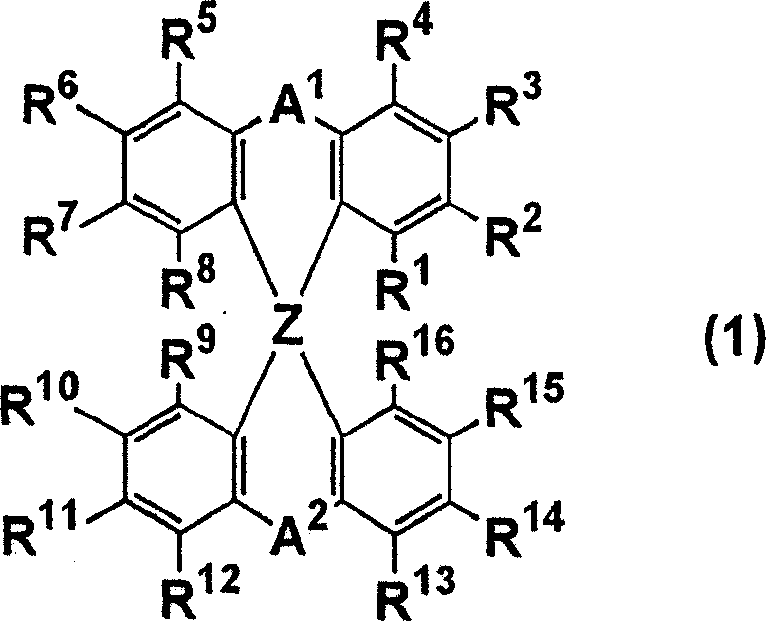
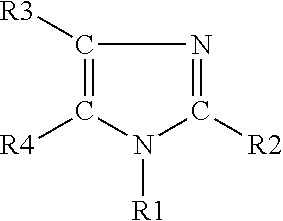
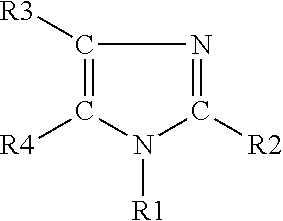
The light emitting device of the present invention relates to a light emitting device which is characterized in that it is a device with an emissive substance present between an anode and cathode, and which emits light by means of electrical energy, and said device has a least one type of compound denoted by (a) to (d) below. (a) A compound having a plurality of 1,7-phenanthroline skeletal structures (b) A benzoquinoline derivative (c) A spiro compound represented by general formula (1) A1 and A2 are each selected from single bonds, substituted or unsubstituted alkyl chains, ether chains, thioether chains, ketone chains and substituted or unsubstituted amino chains. However, A1<> A2. Z represents carbon or silicon. R1 to R16 are each selected from hydrogen, alkyl group, cycloalkyl group, aralkyl group, alkenyl group, cycloalkenyl group, alkynyl group, hydroxyl group, mercapto group, alkoxy group, alkylthio group, aryl ether group, aryl thioether group, aryl group, heterocyclic group, halogen, haloalkane, haloalkene, haloalkyne, cyano group, aldehyde group, carbonyl group, carboxyl group, ester group, carbamoyl group, amino group, nitro group, silyl group, siloxanyl group and a cyclic structure formed with an adjacent substituent. (d) A tetraphenylmethane derivative represented by general formula (2) R17 to R36 are each selected from hydrogen, alkyl group, cycloalkyl group, aralkyl group, alkenyl group, cycloalkenyl group, alkynyl group, hydroxyl group, mercapto group, alkoxy group, alkylthio group, aryl ether group, aryl thioether group, aryl group, heterocyclic group, halogen, haloalkane, haloalkene, haloalkyne, cyano group, aldehyde group, carbonyl group, carboxyl group, ester group, carbamoyl group, amino group, nitro group, silyl group, siloxanyl group and a cyclic structure formed with an adjacent substituent. However, at least one of R17 to R36 is selected from substituents represented by general formula (3). -X-Ar (3) X is a single bond or is selected from the following, and Ar denotes a condensed aromatic ring or heteroaromatic ring. In the case where X is phosphorus oxide, then Ar represents an aromatic hydrocarbon or heteroaromatic ring. n is an natural number.
Owner:TORAY IND INC
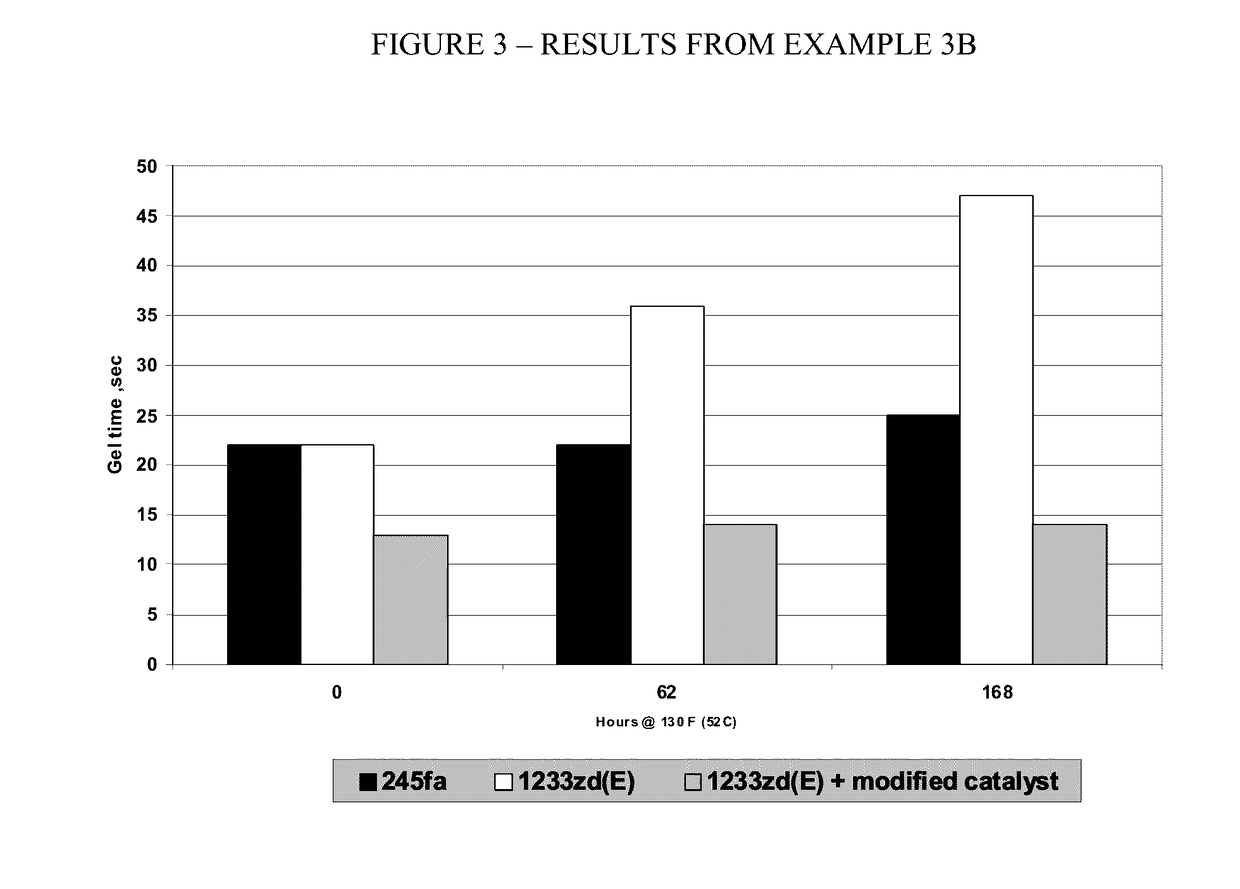
Halogenated alkene heat transfer composition with improved oil return
The invention relates to heat transfer agents and heat transfer compositions containing hydrocarbon lubricating oils and halogentaed alkene heat transfer fluid that promote oil flow and provide for improved oil return. The heat transfer compositions are useful in various heat transfer systems such as refrigeration, cooling, air conditioning, chiller operations.
Owner:ARKEMA INC
Improved stability of polyurethane polyol blends containing halogenated olefin blowing agents
InactiveUS20140051776A1Extended shelf lifeEnhance foam characteristic of foamOther chemical processesPolymer sciencePtru catalyst
A polyol pre-mix composition includes a blowing agent having a halogenated hydroolefin, a polyol, a surfactant, a catalyst composition, and a metal salt. The metal salt may be, for example, a carboxylate, acetylacetonate, alcoholate of a metal selected from the group consisting of Zn, Co, Ca, and Mg. The metal salt may be, for example, a carboxylate and / or alcoholate of a C1-C21 straight chain or branched aliphatic monocarboxylic acid or monoalcohol, such as magnesium formate, zinc octoate, calcium octoate, cobalt octoate, and magnesium octoate, and mixtures thereof. The metal acetylacetonate may be, for example, zinc acetylacetonate, cobalt acetylacetonate, magnesium acetylacetonate, or calcium acetylacetonate. A two-part system for producing a thermosetting foam blend includes (a) a polyisocyanate and, optionally, one or more isocyanate compatible raw materials; and (b) the polyol pre-mix composition. A method for producing a thermosetting foam blend includes combining: (a) a polyisocyanate; and (b) the polyol pre-mix composition.
Owner:ARKEMA INC
Stability of polyurethane polyol blends containing halogenated olefin blowing agents
InactiveUS20140005288A1Easy to prepareLess reactivityOther chemical processesFoaming agentMorpholine
A stable polyol pre-mix composition comprises a blowing agent, a polyol, a surfactant, and a catalyst composition comprising an oxygen-containing amine catalyst. The oxygen-containing amine catalyst may be, an alkanol amine, an ether amine, or a morpholine group. containing compound such as, 2.(2.dimethylaminoethoxy)ethanol or N.N.N′.trimethylaminoethylethanolamine. A stabilized thermosetting foam blend comprises: (a) a polyisocyanate and, optionally, isocyanate compatible raw materials; and (b) a polyol pre. mix composition. A method for stabilizing thermosetting foam blends comprises combining: (a) a polyisocyanate and, optionally, isocyanate compatible raw materials; and (b) a polyol pre. mix composition. A polyurethane or polyisocyanurate foam having uniform cell structure with little or no foam collapse comprises a mixture of: (a) a polyisocyanate and, optionally, one or more isocyanate compatible raw materials; and (b) a polyol pre-mix composition. The resultant polyurethane or polyisocyanurate foams have uniform cell structure with little or no foam collapse.
Owner:ARKEMA INC
Polyurethane foam premixes containing halogenated olefin blowing agents and foams made from same
InactiveUS20140171525A1Faster and improved front-end catalytic reactivityDecreased cream timePolyolFoaming agent
Disclosed are polyol premix compositions, and foams formed therefrom, which comprise a combination of a hydrohaloolefin blowing agent, a polyol, a silicone surfactant, and a catalyst system that includes a bismuth-based metal catalyst. Such catalysts may be used alone or in combination with an amine catalyst and / or other non-amine catalysts.
Owner:HONEYWELL INT INC
Stabilization of foam polyol premixes containing halogenated olefin blowing agents
A storage stable polyol premix compositions, methods of forming such compositions, foamable compositions using the premix compositions, and methods of preparing foams containing the premix compositions, and foams made using the premix composition comprising a polyol component comprising at least 10 wt % of a cashew nutshell liquid based polyol based on a total weight of the polyol component, a tertiary amine catalyst, a silicone surfactant, and a blowing agent comprising a hydrohaloolefin are disclosed.
Owner:HONEYWELL INT INC
Preparation of halo-olefin
ActiveUS7164050B2Increase the effective surface areaIncrease productionPreparation by dehalogenationPreparation by hydrogen halide split-offHaloalkeneSolvent
A process for preparing a halo-olefin to minimize one or more side reactions which form at least one impurity, said process comprising contacting a halogenated hydrocarbon with a metal dehalogenating agent dissolved in a solvent under conditions sufficient to dehalogenate said halogenated hydrocarbon to produce a product stream comprising said halo-olefin and at least one impurity, said metal dehalogenating agent having an average particle size within a range of average particle sizes, said impurity concentration of said product stream being essentially constant within said range and increasing significantly below said range.
Owner:HONEYWELL INT INC
High-valent metal fluorination catalyst and preparation method and application thereof
ActiveCN109999788AIncrease temperatureHigh catalytic activityPreparation by halogen replacementMetal/metal-oxides/metal-hydroxide catalystsChemical synthesisAlkane
The invention discloses 'a high-valent metal fluorination catalyst and a preparation method and application thereof', and belongs to the field of chemical synthesis. The high-valent metal fluorinationcatalyst is composed of high-valent metal ions and auxiliary agents, wherein the mass percentages of high-valent metal ions and auxiliary agents are 70%-100% and 0-30% in sequence. A 'fluorine storage material' is introduced in an oxidation stage to convert low-valent metal ions into high-valent metal ions. The high-valent metal fluorination catalyst has high use temperature, high catalytic activity and long service life, and is mainly used for preparing a fluorine-containing alkane through a fluorine-chlorine exchange reaction of gas-phase catalytic halogenated alkane and hydrogen fluoride under high temperature or preparing a fluorine-containing olefin through a fluorine-chlorine exchange reaction of gas-phase catalytic halogenated olefin and hydrogen fluoride under high temperature.
Owner:泉州宇极新材料科技有限公司
Chromium base catalyst, and preparation method and use thereof
InactiveCN104907063AInhibit carbon depositionCatalytic activity unchangedPreparation by hydrogen halide split-offPreparation by halogen replacementCalcium silicateChemical synthesis
The invention discloses a chromium base catalyst, and a preparation method and a use thereof, and belongs to the field of chemical synthesis. The precursor of the catalyst is composed of 90-99.9mass% of a trivalent chromium compound and 0.1-10mass% of a silicon-containing compound, wherein the trivalent chromium compound is chromic oxide or chromium hydroxide, and the silicon-containing compound can be zinc silicate, nickel silicate, magnesium silicate, aluminum silicate, zirconium silicate, barium silicate, bismuth silicate, ammonium fluorosilicate, nickel hexafluorosilicate, zinc fluorosilicate, magnesium fluorosilicate, aluminum fluorosilicate or calcium fluorosilicate The chromium base catalyst has the advantages of large specific surface area, effective inhibition of carbon formation, and long service life, is mainly used for gas phase catalysis of reactions for preparing hydrofluorocarbons or fluorinated alkenes from halogenated hydrocarbons or halogenated alkenes at a high temperature, and can also be used for gas phase catalysis of reactions for preparing hydrofluoroalkenes through hydrogen fluoride removal of hydrofluorocarbons at a high temperature.
Owner:BEIJING YUJI SCI & TECH
Cross-metathesis reaction of functionalized and substituted olefins using group 8 transition metal carbene complexes as metathesis catalysts
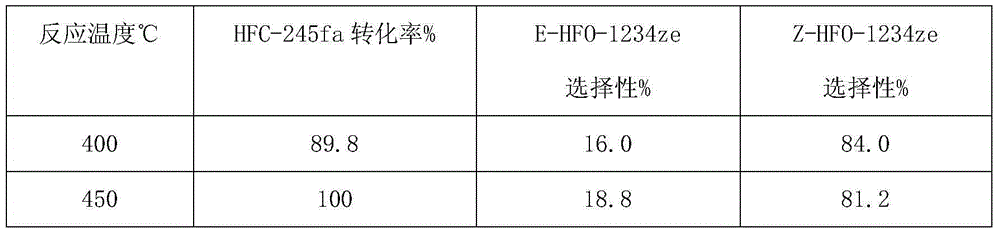
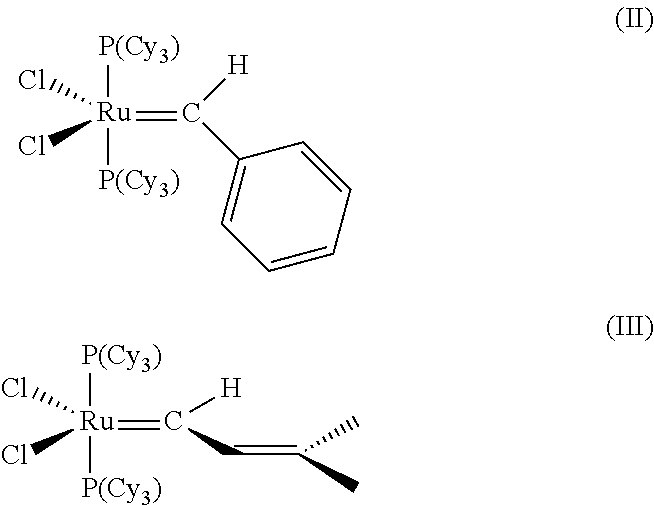
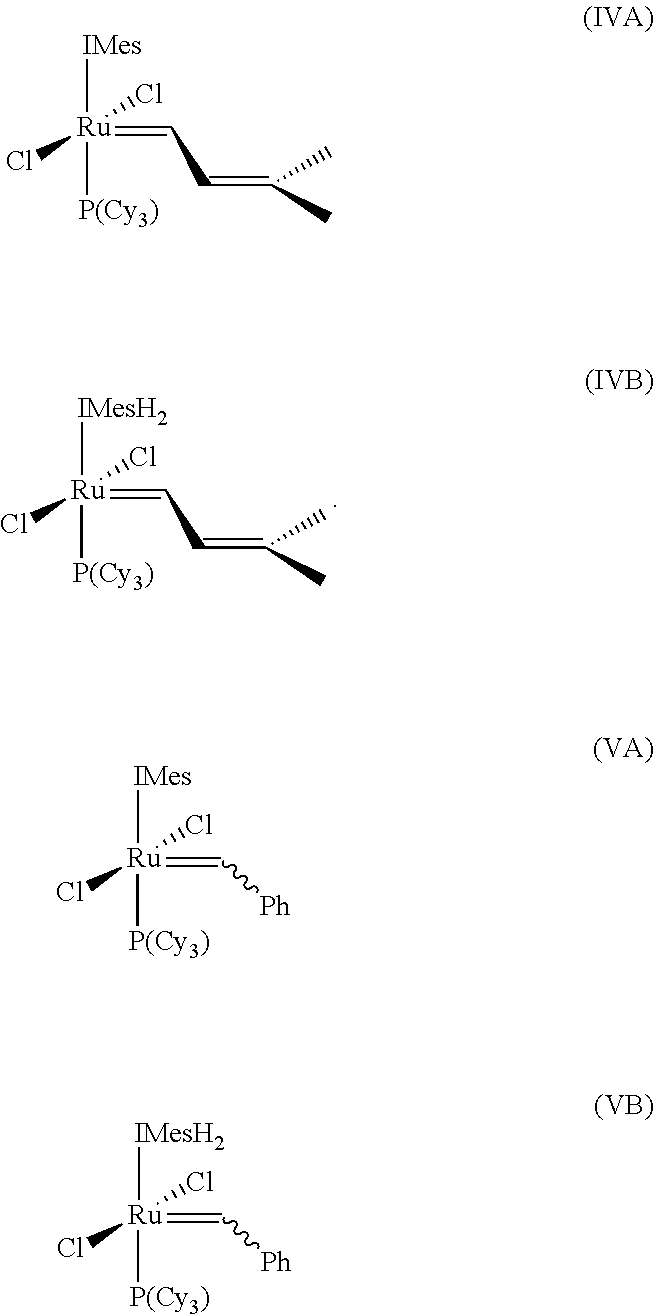
InactiveUS20140288319A1Hydroxy compound preparationHydrocarbon by metathesis reactionHaloalkenePtru catalyst
The invention pertains to the use of Group 8 transition metal carbene complexes as catalysts for olefin cross-metathesis reactions. In particular, ruthenium and osmium alkylidene complexes substituted with an N-heterocyclic carbene ligand are used to catalyze cross-metathesis reactions to provide a variety of substituted and functionalized olefins, including phosphonate-substituted olefins, directly halogenated olefins, 1,1,2-trisubstituted olefins, and quaternary allylic olefins. The invention further provides a method for creating functional diversity using the aforementioned complexes to catalyze cross-metathesis reactions of a first olefinic reactant, which may or may not be substituted with a functional group, with each of a plurality of different olefinic reactants, which may or may not be substituted with functional groups, to give a plurality of structurally distinct olefinic products. The methodology of the invention is also useful in facilitating the stereoselective synthesis of 1,2-disubstituted olefins in the cis configuration.
Owner:CALIFORNIA INST OF TECH
Copolymer and method for producing the same
ActiveUS20100331479A1Reduce impactSufficient dynamic water repellencyGroup 4/14 element organic compoundsThin material handlingMethacrylateHaloalkene
To provide a copolymer which can impart sufficient dynamic water repellency, after air-drying water repellency and friction durability to a surface of an article, and which has low impact on the environment, a method for producing the same, a water repellent composition and an article excellent in dynamic water repellency, after air-drying water repellency and friction durability.A copolymer which is characterized in that it has structural units based on the following monomer (a) and structural units based on the following monomer (b), wherein the content of structural units based on the monomer (a) is from 5 to 95 mass % in the total structural units (100 mass %), the content of structural units based on the monomer (b) is from 5 to 60 mass % in the total structural units (100 mass %) and the sum of the contents of structural units based on the monomer (a) and structural units based on the monomer (b) is at least 65 mass % in the total structural units (100 mass %), and a water repellent composition which comprises the copolymer: Monomer (a): a (meth)acrylate having no Rf group and having a C20-30 alkyl group; Monomer (b): a halogenated olefin.
Owner:ASAHI GLASS CO LTD
Process for the preparation of fluorinated olefin
InactiveUS6211420B1Increase productionHigh yieldPreparation by halogen replacementHalogenDouble bond
In a process for preparing a fluorinated olefin having a carbon-carbon double bond, the carbon atoms of which have a fluorine atom, by reacting a halogenated olefin having at least one carbon-carbon double bond, a carbon atom or the carbon atoms of which bond have a chlorine atom or atoms bound thereto, and, in which the carbon atom or atoms with a single bond in the molecule have no halogen atom other than a fluorine atom, with an alkali metal fluoride, said reaction of the halogenated olefin with the alkali metal fluoride is conducted in the presence of an organic halogen-containing compound having a carbon-carbon single bond, a carbon or the carbons of which have at least one halogen atom other than fluorine atom.
Owner:NAT INST OF ADVANCED IND SCI & TECH +1
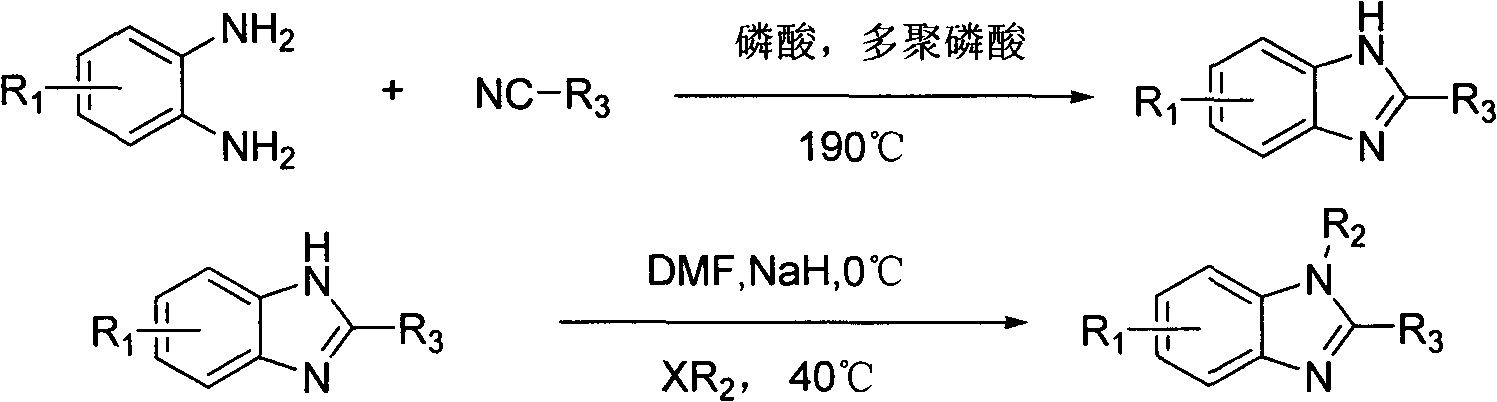
1-alkyl-2-substituted phenyl benzimidazole compound synthesis method and application
The invention provides a 1-alkyl-2-substituted phenyl benzimidazole compound organic light-emitting material as well as a preparation method and application thereof. The preparation method comprises the following steps of: firstly, carrying out condensation reaction on an o-phenylenediamine compound and an aromatic cyanogens compound under the catalytic action of phosphoric acid and polyphosphoric acid to obtain a series of 2-substituted phenyl benzimidazole compounds; and reacting doubled 2-substituted phenyl benzimidazole compounds with 1.0-1.5 times of halogenated hydrocarbon or halogenated olefin under the action of 1.0-6.0 times of sodium hydride for 4-24 hours at 10-80 DEG C to obtain a series of 1-alkyl-2-substituted phenyl benzimidazole compounds. The 1-alkyl-2-substituted phenyl benzimidazole compounds can be used as a light-emitting layer or electronic transmission layer of an organic light-emitting diode.
Owner:NANJING UNIV OF TECH
Blowing agents, foam premixes and foams containing halogenated olefin blowing agent and adsorbent
InactiveUS20130210946A1Reduced stabilityReduce contentOrganic chemistryPolyisocyanurateBlowing agent
The invention provides polyurethane and polyisocyanurate foams and methods for the preparation thereof. More particularly, the invention relates to closed-celled, polyurethane and polyisocyanurate foams and methods for their preparation. Preferably, the foams are produced with a polyol premix composition which comprises a combination of a hydrohaloolefin blowing agent, a polyol, a catalyst and an adsorbent material.
Owner:HONEYWELL INT INC
Systems For Efficient Heating And/Or Cooling And Having Low Climate Change Impact
ActiveUS20120012591A1Avoid flowThermal insulationDomestic cooling apparatusHaloalkeneThermal insulation
A heat transfer system and method for controlling heat transfer to and from a container having thermal insulation disposed with respect to said container or compartment so as to inhibit the flow of heat into and / or out of the compartment, said insulation comprising a polymeric material having closed cells therein wherein said cells are formed from and / or contain a haloalkene according to Formula IA:where each R is independently Cl, F, H, or CF3, provided that the total number of carbon atoms is either 3 or 4,R′ is (CR2)nY,Y is CF3 and n is 0 or 1;and a heat transfer system for adding and / or removing heat from the compartment or container by use of a heat transfer fluid comprising a haloalkene Formula IB:where each R is independently Cl, F or HR′ is (CR2)nY,Y is CF3 and n is 0 or 1.
Owner:HONEYWELL INT INC
Halogenated olefin combined solvent containing 1-chloro-2, 3, 3-trifluoropropene and application thereof
The invention relates to the technical field of fluorine-containing cleaning agents, and provides a halogenated olefin combined solvent containing 1-chloro-2, 3, 3-trifluoropropene. The halogenated olefin combined solvent is prepared from the following components in parts by mass: 3 to 90 parts of 1-chloro-2, 3, 3-trifluoropropene, 5 to 95 parts of trans-1, 2-dichloroethylene; and 0.2 to 5 parts of methyl perfluoroheptene ether. According to the invention, the synergistic effect of the three substances is utilized; azeotrope or approximate azeotrope can be formed, the obtained combined solventis stable, free of flash point, low in toxicity and corrosivity, good in material compatibility, good in dissolving and removing capacity for pollutants and high in volatilization drying speed, the ODP value (ozone consumption potential value) is zero, and the GWP value (global warming potential value) is extremely low. The invention also provides an application of the halogenated olefin combinedsolvent in a cleaning agent, a diluent and an aerosol.
Owner:SHANGHAI RICHEM ENVIROTEC CO LTD
Preparation method for polymer carbon material
The invention discloses a preparation method for a polymer carbon material, and belongs to the technical field of chemical synthetic carbon materials. The preparation method is as follows: on the basis of taking alkaline metal or alkaline earth metal and polyhalogenated olefin, halogenated polyolefin, polyhalogenated hydrocarbon or a derivative thereof as raw materials, carrying out metal coupling reaction in a body of polyhalogenated olefin, halogenated polyolefin, polyhalogenated hydrocarbon or the derivative thereof or the good solvent under an inert environment; after the reaction, utilizing halogen or a reactive hydrogen compound to remove residual metals; washing by use of an organic solvent, pickling, washing, filtering and drying to obtain the polymer porous carbon material. The preparation method disclosed by the invention has the advantages of being simple in equipment, easy to operate, low in cost, less in byproduct, high in yield, good in adsorption, and the like; the polymer carbon material prepared by the preparation method disclosed by the invention can be used as corrosion-resistant wear-resisting, conductive, capacitive, adsorbent, oil-absorbing and hydrogen-storing materials.
Owner:SHANDONG UNIV OF TECH
Preparation method for diaryl diazonium methane derivative containing double bonds and application thereof
ActiveCN105418451AMild reaction conditionsLow costOrganic free radical generationBulk chemical productionTosylCarbene
The present invention relates to a preparation method for a diaryl diazonium methane derivative containing double bonds and an application thereof. The present invention aims to solve the problem that a stable carbene reactive intermediate with functional groups cannot be prepared from inexpensive chemistry reagents under mild conditions in the prior art. The method comprises the following steps: taking 4,4'-dihydroxybenzophenone as a raw material, performing substitution reaction on the 4,4'-dihydroxybenzophenone and halogenated olefin, performing hydrazonization reaction on the 4,4'-dihydroxybenzophenone and p-toluenesulfonohydrazide, and removing a p-toluenesulfonyl protecting group under the alkaline condition to obtain the diaryl diazonium methane derivative containing double bonds. According to the preparation method for the diaryl diazonium methane derivative containing double bonds and the application thereof, the diaryl diazonium methane derivative containing double bonds is used as a precursor for preparing the carbene.
Owner:HARBIN INST OF TECH
Copolymer, method for its production, and water/oil repellent composition
InactiveUS20110071248A1Excellent in water repellency repellencyExcellent in repellency oil repellencyFibre treatmentHaloalkenePolymer science
To provide a copolymer which is capable of providing an article surface with a dynamic water repellency and which has extremely excellent washing durability of water repellency and oil repellency, while placing little burden on the environment; a method for its production; and a water / oil repellent composition.A copolymer comprising structural units of the monomer (a) and structural units of the monomer (b), wherein the total of the content of structural units based on the monomer (a) and the content of structural units based on the monomer (b) is at least 85 mass % of all structural units (100 mass %), and the molar ratio ((a) / (b)) of the content of structural units based on the monomer (a) to the content of structural units based on the monomer (b) is from 0.12 to 3; and a water / oil repellent composition containing such a copolymer. Monomer (a): a compound represented by (Z—Y)n—X, wherein Z is a C1-6 polyfluoroalkyl group or the like, Y is a bivalent organic group or a single bond, n is 1 or 2, and when n is 1, and X is a polymerizable unsaturated group. Monomer (b): a halogenated olefin.
Owner:ASAHI GLASS CO LTD
Haloolefin-based composition and use thereof
ActiveUS10731065B2Easily cause oxidizationImprove stabilityOther chemical processesTransportation and packagingHaloalkeneThermodynamics
Owner:DAIKIN IND LTD
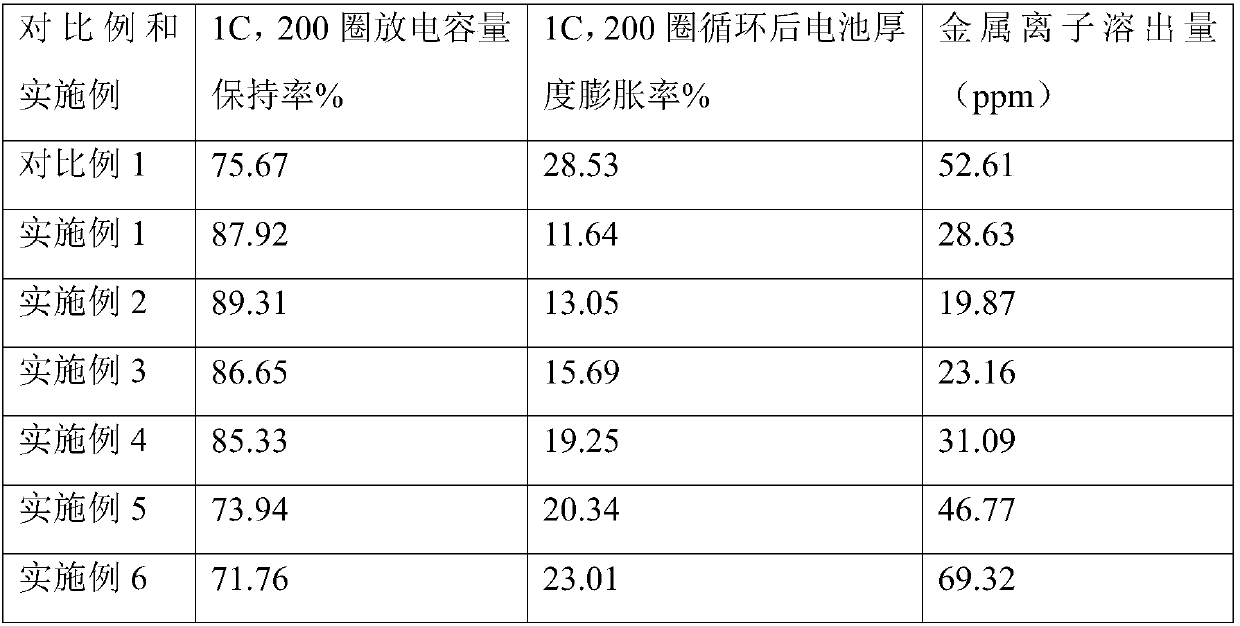
Non-aqueous electrolyte and secondary lithium battery
ActiveCN111162316ADissolution inhibitionReduce thickness swellSecondary cells servicing/maintenanceOrganic electrolytesElectrolytic agentPhenyl Ethers
The invention relates to a non-aqueous electrolyte. The electrolyte comprises lithium salt, an organic solvent and a functional additive, the functional additive comprises a triphenylphosphine derivative, and the structural general formula of the triphenylphosphine derivative is shown in the specification, wherein R1, R2 and R3 are independently selected from hydrogen, hydroxyl, halogen, alkyl, alkoxy, halogenated alkyl, halogenated alkoxy, alkenyl, halogenated alkenyl, phenyl, halogenated phenyl, biphenyl, halogenated biphenyl, phenyl ether, triphenyl, halogenated phenyl ether, halogenated triphenyl, amino, ester or cyano, the halogen is F, Cl or Br, and the halogenation is partial substitution or complete substitution. The electrolyte disclosed by the invention can well inhibit the dissolution of metal ions in the positive electrode material, protect the positive electrodes, effectively reduce the thickness expansion of the lithium battery and improve the capacity retention ratio ofthe secondary lithium battery.
Owner:ZHANGJIAGANG GUOTAI HUARONG NEW CHEM MATERIALS CO LTD
Catalysts for polyurethane foam polyol premixes containing halogenated olefin blowing agents
The invention provides polyurethane and polyisocyanurate foams and methods for the preparation thereof. More particularly, the invention relates to closed-celled, polyurethane and polyisocyanurate foams and methods for their preparation. The foams are characterized by a fine uniform cell structure and little or no foam collapse. The foams are produced with a polyol premix composition which comprises a combination of a hydrohaloolefin blowing agent, a polyol, a silicone surfactant, and a non-amine catalyst used alone or in combination with an amine catalyst.
Owner:HONEYWELL INT INC
1-haloalkadiene and process for preparing same and process for preparing (9e,11z)-9,11-hexadecadienyl acetate
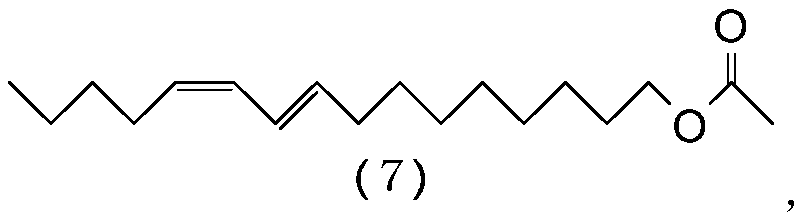
ActiveCN110386855AHigh yieldHigh purityOrganic compound preparationPreparation by ester-hydroxy reactionHaloalkeneAryl
The object of the invention is to prepare (9E,11Z)-9,11-hexadecadienyl acetate with a good yield and high purity. The present invention provides a process for preparing a 1-haloalkadiene of the general formula (1): CH-(CH)-CH=CH-CH-CH-(CH)-X, comprising a step of conducting a Wittig reaction between a haloalkenal of the general formula (2): OHC-CH=CH-(CH)-X, and a triarylphosphonium pentylide of the general formula (3): CH-(CH)-CH-PAr, to obtain the 1-haloalkadiene, and the use of a (7E,9Z)-1-halo-7,9-tetradecadiene obtained by the process for a process of preparing (9E,11Z)-9,11-hexadecadienyl acetate.
Owner:SHIN ETSU CHEM IND CO LTD
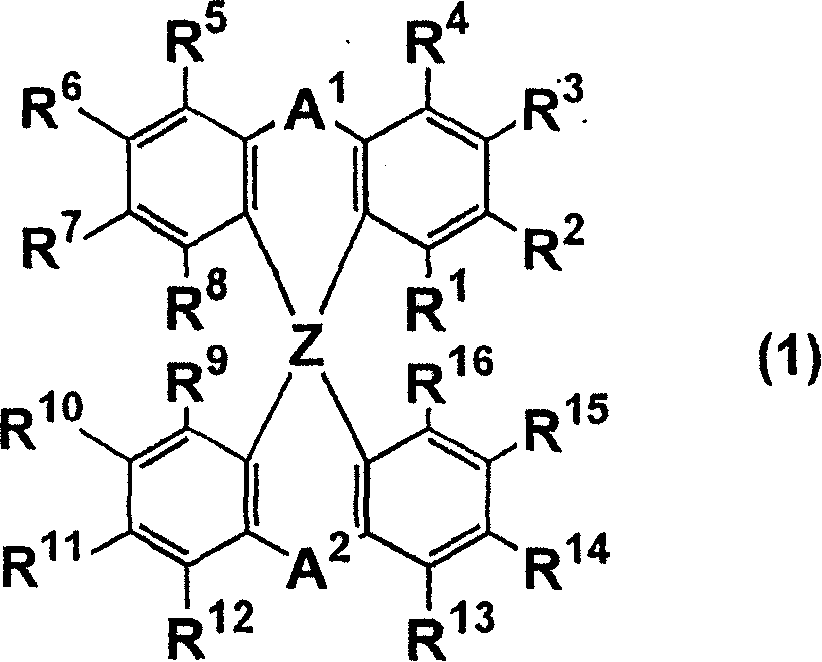
Luminescent element material and luminescent element comprising the same
The light emitting device of the present invention relates to a light emitting device which is characterized in that it is a device with an emissive substance present between an anode and cathode, and which emits light by means of electrical energy, and said device has a spiro compound represented by general formula (1) A1 and A2 are each selected from single bonds, substituted or unsubstituted alkyl chains, ether chains, thioether chains, ketone chains and substituted or unsubstituted amino chains. However, A1<> A2. Z represents carbon or silicon. R1 to R16 are each selected from hydrogen, alkyl group, cycloalkyl group, aralkyl group, alkenyl group, cycloalkenyl group, alkynyl group, hydroxyl group, mercapto group, alkoxy group, alkylthio group, aryl ether group, aryl thioether group, aryl group, heterocyclic group, halogen, haloalkane, haloalkene, haloalkyne, cyano group, aldehyde group, carbonyl group, carboxyl group, ester group, carbamoyl group, amino group, nitro group, silyl group, siloxanyl group and a cyclic structure formed with an adjacent substituent.
Owner:TORAY IND INC
Surface treatment agent
ActiveUS20190077984A1Easy to waterExcellent oil-repellencyOther chemical processesLiquid repellent fibresWater basedLiquid medium
Provided is a surface treatment agent which does not use fluorine-containing monomers, particularly fluoroalkyl group-containing monomers. The surface treatment agent is a water-based emulsion which includes: a copolymer (1) which includes a first polymer formed from first monomers, and a second polymer formed from second monomers, wherein the second polymer is polymerized in the presence of the first polymer, the first monomers include a long-chain acrylate ester monomer (a) represented by the formula CH2═CA11-C(═O)—O-A12 (in the formula, A11 represents hydrogen, a monovalent organic group, or a halogen, and A12 represents a C18-30 straight-chain or branched hydrocarbon group), the first monomers do not include a halogenated olefin monomer (b), and the second monomers include the halogenated olefin monomer (b); a surfactant (2) including a nonionic surfactant; and a liquid medium (3) including water.
Owner:DAIKIN IND LTD
Preparation method of fabric with high chemical protection property
PendingCN114161792AHigh barrierReduced quality per unit areaLamination ancillary operationsSynthetic resin layered productsHaloalkenePolymer science
The invention discloses a preparation method of a fabric with high chemical protection performance. According to the invention, a PTFE film and a PPS fabric are subjected to hot-pressing compounding at 80 DEG C, a polymer modified film coated on the surface of the PPS fabric is fused at the temperature, the PTFE film and the polymer modified film are entangled among molecules at high temperature and are transferred along with the molecules, and finally, high-temperature hot-pressing compounding of two interfaces of PTFE and PPS is achieved, so that a skeleton layer is obtained; the polytetrafluoroethylene (PTFE) film is excellent in acid and alkali resistance, so that the PTFE film is selected to coat the surface of the PPS fabric, the prepared fabric has higher acid and alkali corrosion resistance, meanwhile, the polyhalogenated olefin film is selected as a barrier layer material of the chemical protection fabric, the film is compact in structure and excellent in chemical stability, and due to the fact that the film contains halogen elements, the film is nonflammable, and the chemical protection fabric has the advantages of being high in acid and alkali corrosion resistance. The combustion characteristic is carbonization, and no molten drops occur. When the film is used as a barrier layer of the chemical protection fabric, the chemical protection capability of the whole fabric is greatly improved.
Owner:邦威防护科技股份有限公司
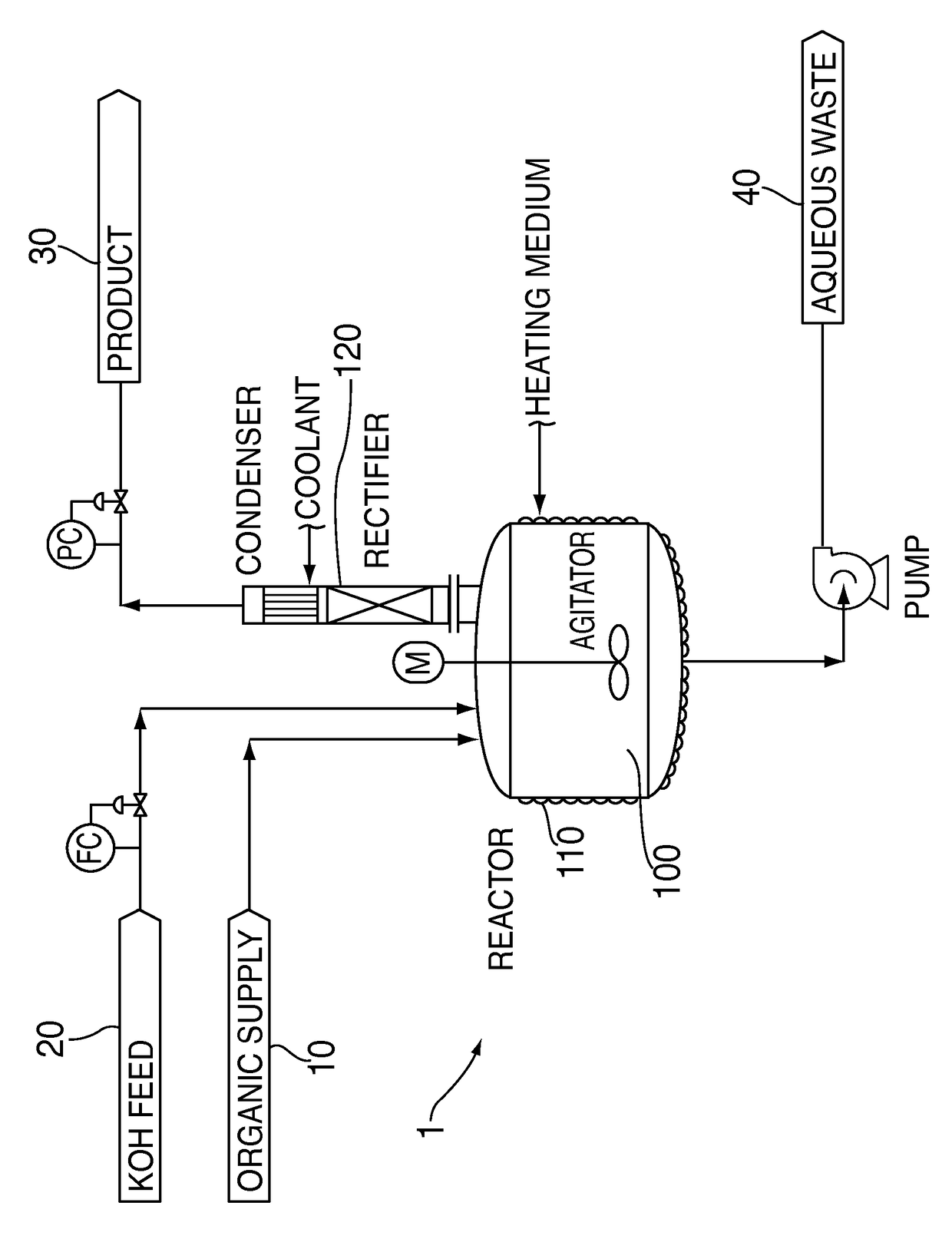
Process for dehydrohalogenation of halogenated alkanes
ActiveUS9884796B2High selectivityHigh yieldPreparation by dehalogenationPreparation by hydrogen halide split-offAlkaneHaloalkene
A process for the manufacture of halogenated olefins in semi-batch mode by dehydrohalogenation of halogenated alkanes in the presence of an aqueous base such as KOH which simultaneously neutralizes the resulting hydrogen halide. During the process, aqueous base is continuously added to the haloalkane which results in better yields, lower by-product formation and safer / more controllable operation.
Owner:HONEYWELL INT INC
Tobacco shred expanding agent
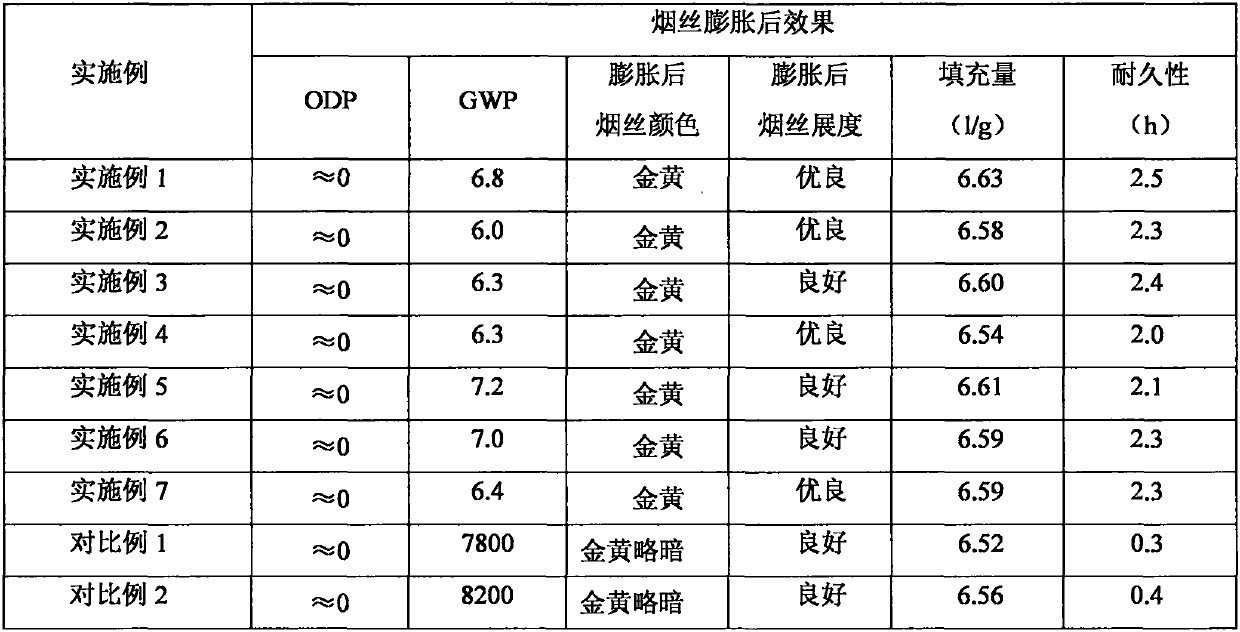
The invention relates to a tobacco shred expanding agent. The tobacco shred expanding agent comprises the following components in percentage by mass: 49-98% of halogenated olefin; 1-50% of an ester compound; and 1-20% of halogenated ether or halogenated alcohol. The tobacco shred expanding agent provided by the invention has the advantages of the zero ODP value, the very low GWP value, the moderate boiling point, safety, low toxicity, incombustibility and volatility, and is very suitable for expanding tobacco shreds in the tobacco processing industry.
Owner:BEIJING INST OF AEROSPACE TESTING TECH
Lewis acid-base pair catalyst, preparation method and method for catalytically synthesizing polyester
The invention relates to the field of catalyst synthesis. Aiming at the problems of lack of existing catalyst types as well as low polymer molecular weight, low initiation efficiency, existence of side reaction and low monomer conversion rate in polyester generation through phthalic anhydride and cyclohexene oxide bulk copolymerization, the invention provides a Lewis acid-base pair which has a structure shown in the specification, wherein n is equal to 1, 2, 3 or 4, and X is equal to Cl, Br or I. The preparation method of the Lewis acid-base pair catalyst comprises the following steps of: preparing quaternary phosphonium salt from triphenylphosphine and halogenated olefin; and preparing the catalyst from the quaternary phosphonium salt and 9-boron bicyclo [3.3.1] nonane. The invention also provides a method for synthesizing polyester, which comprises the step of: by taking anhydride and epoxy monomer as raw materials, carrying out ring-opening polymerization under the catalysis of the Lewis acid-base pair catalyst to generate an alternating copolymer. The catalytic system has the advantages of simplicity in preparation, high activity, convenience in use, low cost and wide applicability, and is very suitable for industrial production.
Owner:QINGDAO UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com