Lewis acid-base pair catalyst, preparation method and method for catalytically synthesizing polyester
A Lewis acid-base pair and catalyst technology, which is applied in the field of Lewis acid-base pair catalyst, catalyzed synthesis of polyester, and preparation, can solve the problems of low polymer molecular weight, lack of catalyst types, low monomer conversion rate, etc., and achieve improved reaction Poor control, low price, high activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
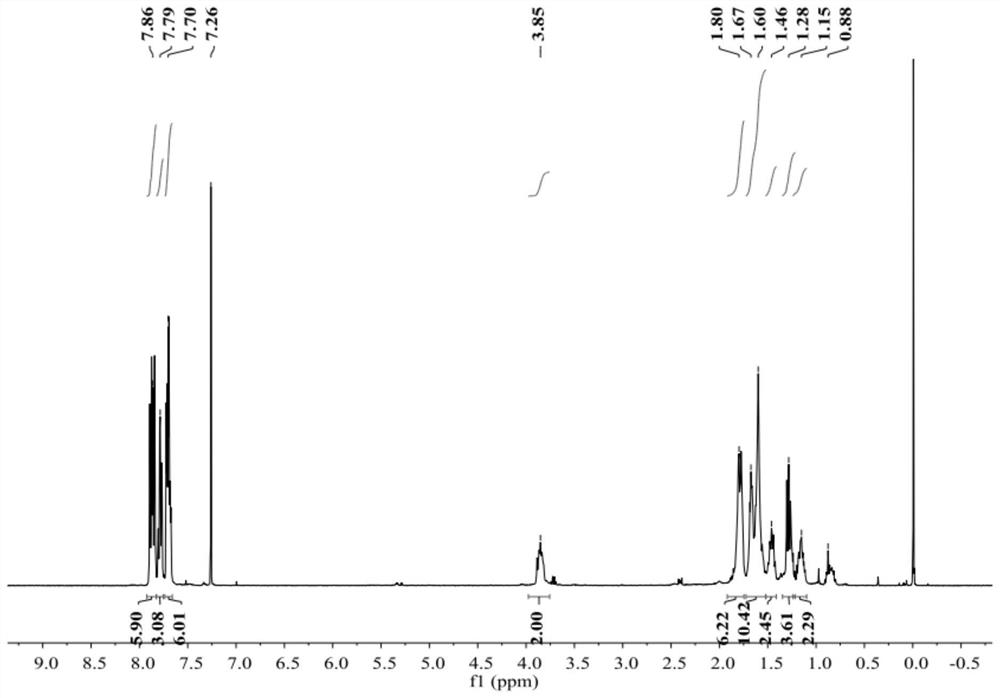
preparation example 11~15
[0057] Preparation example 1.1~1.5 prepares quaternary phosphorus salt
[0058] Select triphenylphosphine and halogenated olefins to prepare quaternary phosphorus salts. The specific operations of Preparation Examples 1.1-1.5 are as follows.
preparation example 11
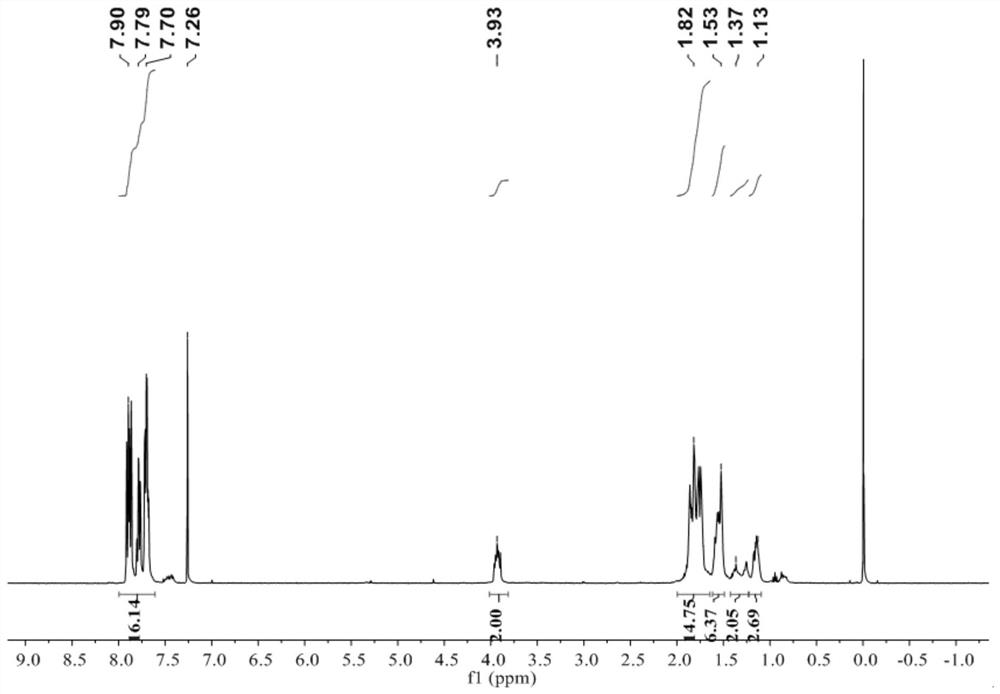
[0060] Triphenylphosphine (0.262 g, 1 mmol, 1 equiv) and 3-bromopropane (0.13 mL, 1.5 mmol, 1.5 equiv) were dissolved in toluene (5 mL) and heated at 70 °C for 48 h. The reaction mixture was concentrated in vacuo to obtain the crude product, which was further purified by washing with ether three times. The white solid was collected by vacuum filtration and dried under vacuum at 40 °C for 12 hours.
preparation example 12
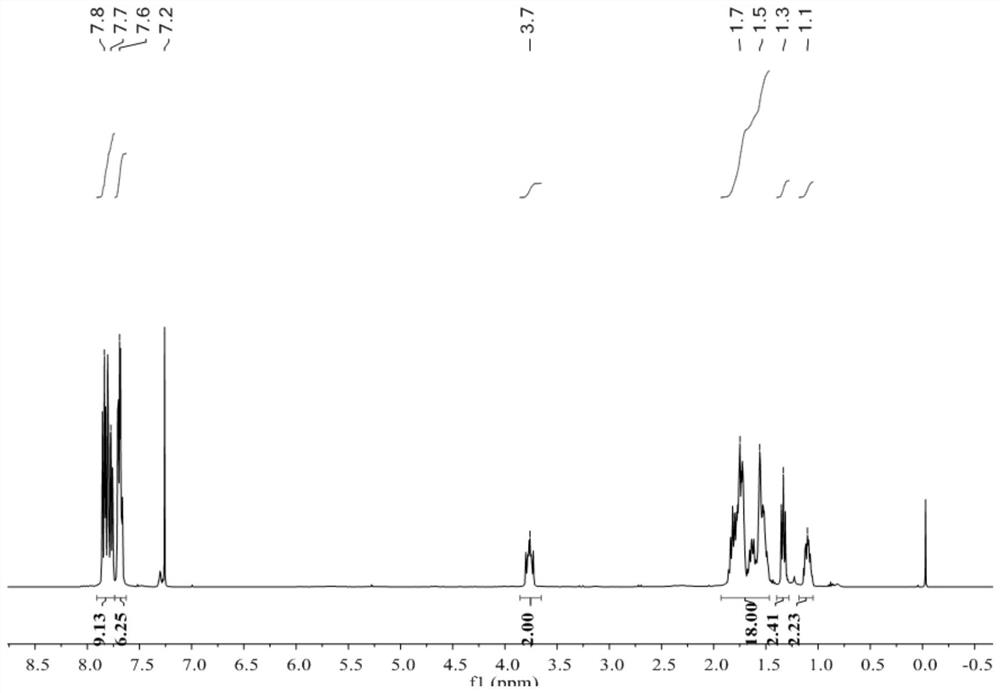
[0062] Triphenylphosphine (0.262 g, 1 mmol, 1 equiv) and 4-bromo-1-butene (0.15 mL, 1.5 mmol, 1.5 equiv) were dissolved in toluene (5 mL) and heated at 70°C for 48 hours. The reaction mixture was added three times the solvent volume of diethyl ether, and placed in the refrigerator to freeze. The white solid was filtered by vacuum while cooling and washed three times with diethyl ether. The white solid was dried under vacuum at 40 °C for 12 h.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com