Polyurethane foam premixes containing halogenated olefin blowing agents and foams made from same
a technology of halogenated olefin and polyurethane, which is applied in the field of polyurethane and polyisocyanurate foams, can solve the problems of undesirable increase in reactivity time, inconvenient use, and undesirable effects, and achieves the effect of improving the cream time, improving the front-end catalytic reactivity, and improving the cream tim
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
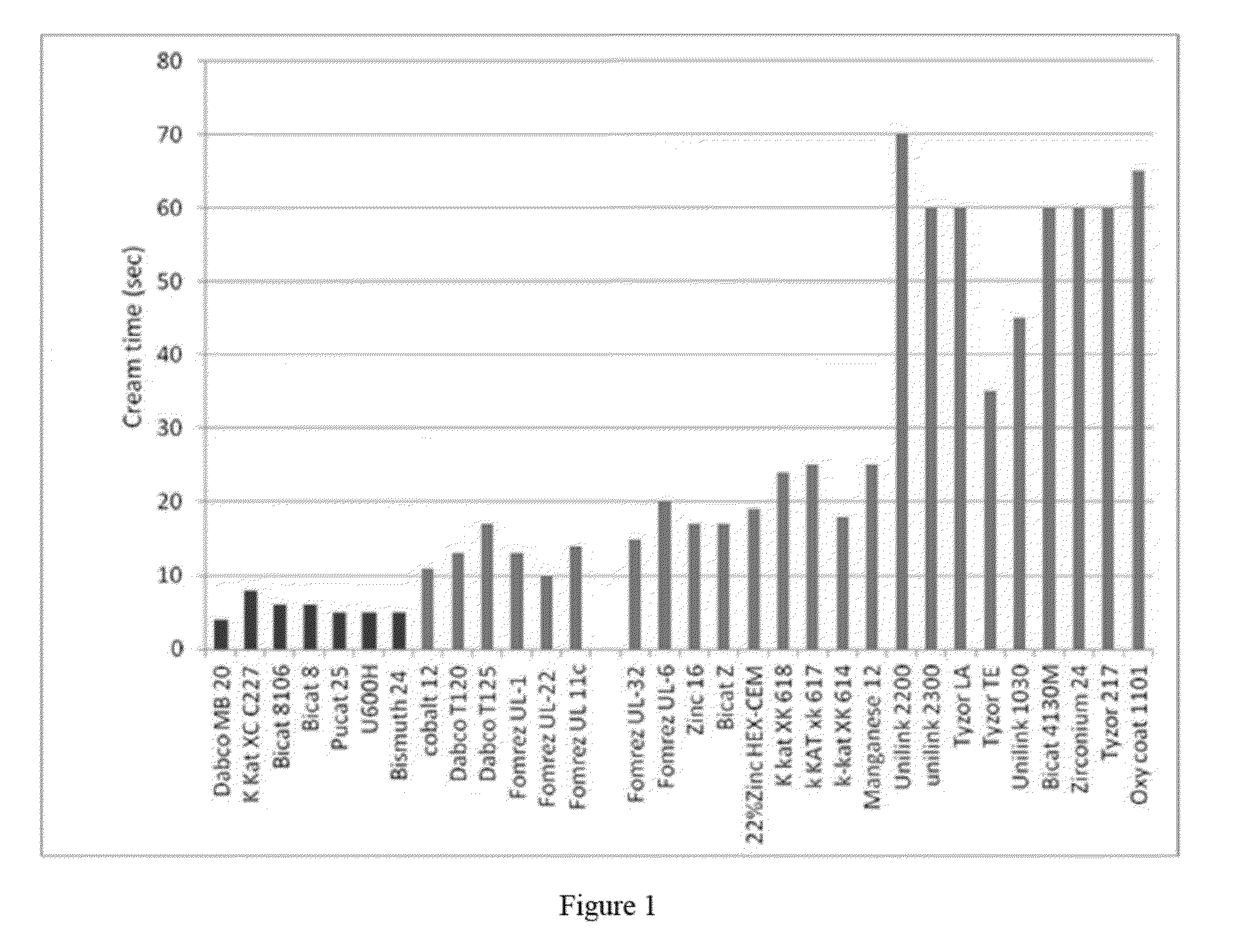
[0055]To evaluate the front-end reactivity of metal catalysts, a resin with a formulation in Table 1 was prepared. Various metal catalysts, including bismuth, cobalt, tin, zinc, manganese, titanium, zirconium iron, were tested in the polyol preblend. The polyol blend (50° F.) was then reacted with equal amount of isocyanate Lupranate M20 at 70° F. The cream time was recorded and was based on the interval of time between mixing together the polyol and diisocyante and the change in the color of the liquid as the mixture begins to rise.
TABLE 1ComponentPhppTerate 402060Voranol 470X30Voranol 36010Antiblaze AB 8010PHT-4-Diol3Water2.5Dabco DC 1931.51233zd (E)12Metal catalyst3
[0056]As evident from FIG. 1, the first seven catalysts tested (all bismuth-based catalysts) all have cream times below those of the other metal catalysts tested. Only bismuth catalysts showed a cream time of around 5 seconds. All other metal catalysts produced a reaction with a cream time longer than 10 seconds. This ...
example 2
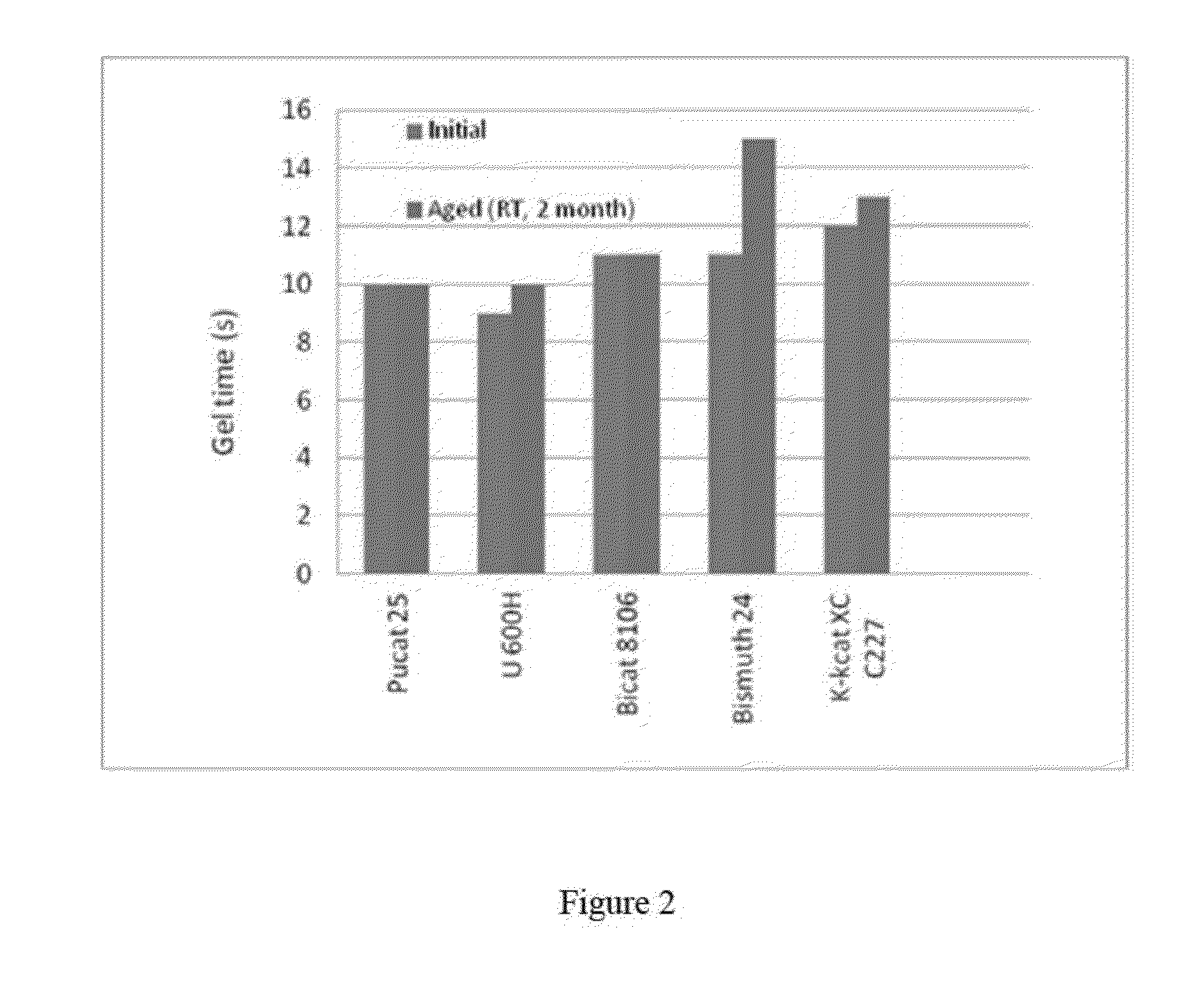
[0057]Bismuth catalysts also display good stability in well-designed resin system. The following experiments which used Toyocat DM70 as the gelling catalysts which is not a front-end catalyst, and Dabco K15 as the trimer catalyst which is good for back-end cure, along with the bismuth catalysts (Table 2).
[0058]The initial reactivity of such resin system was measured by reacting the freshly prepared resin at 50° F. with equal amount of isocyanate Lupranate M20 at 70° F. The aged reactivity was measured similarly, e.g. by reacting the resin (50° F.) which has been aged at room temperature for a predetermined time, with Lupranate M20 at 70° F.
TABLE 2ComponentPhppTerate 402060Voranol 470X30Voranol 36010Antiblaze AB 8010PHT-4-Diol3Water2.2Dabco DC 1931.5Toyocat DM 703K 151Bismuth catalyst0.51233zd (E)12
[0059]As illustrated in FIG. 2, the results showed bismuth catalysts such as Pucat 25, U600H, Bicat 8106, K-KAT XC C227 has shown excellent stability in the catalyst package used the resin...
example 3
[0060]Example 2 is repeated using Dabco MB20, Bicat 8, and Bicat 8210. The initial reactivity of such resin system is measured by reacting the freshly prepared resin at 50° F. with equal amount of isocyanate Lupranate M20 at 70° F. The aged reactivity is measured similarly, by reacting the resin (50° F.) which is aged at room temperature for a predetermined time, with Lupranate M20 at 70° F.
[0061]The results show that the bismuth catalysts Dabco MB20, Bicat 8, and Bicat 8210 exhibit stability in the catalyst package that is within commercially tolerable levels and also that reactivity is maintained after aging.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com