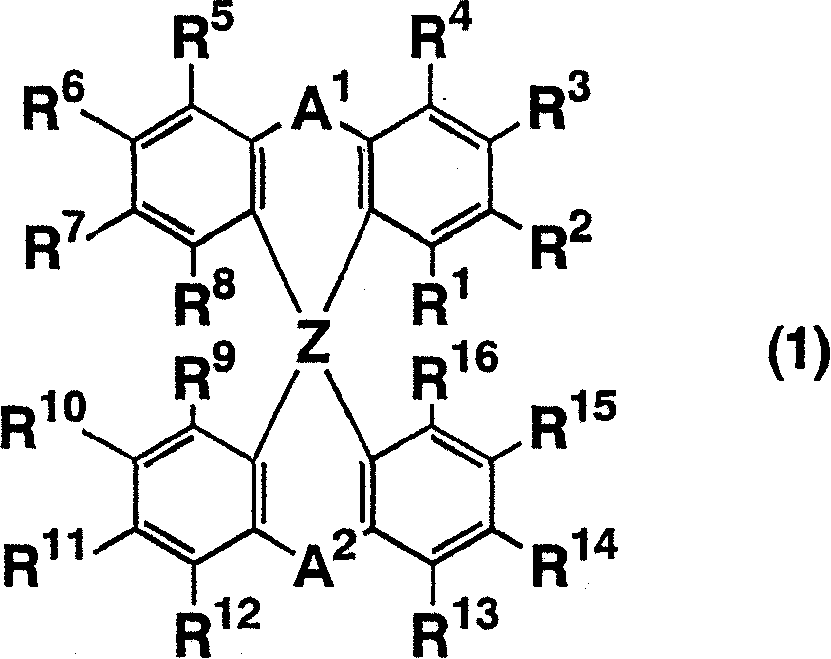
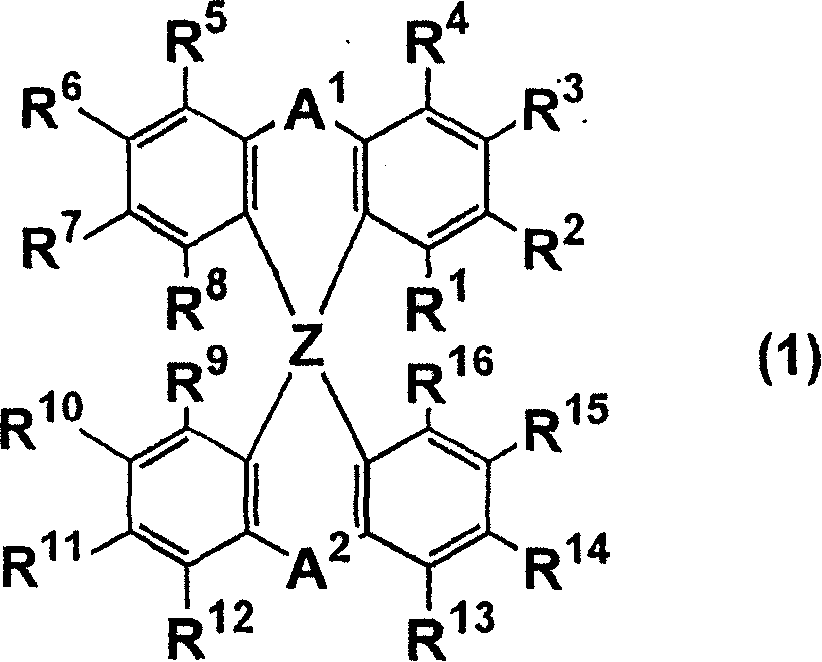
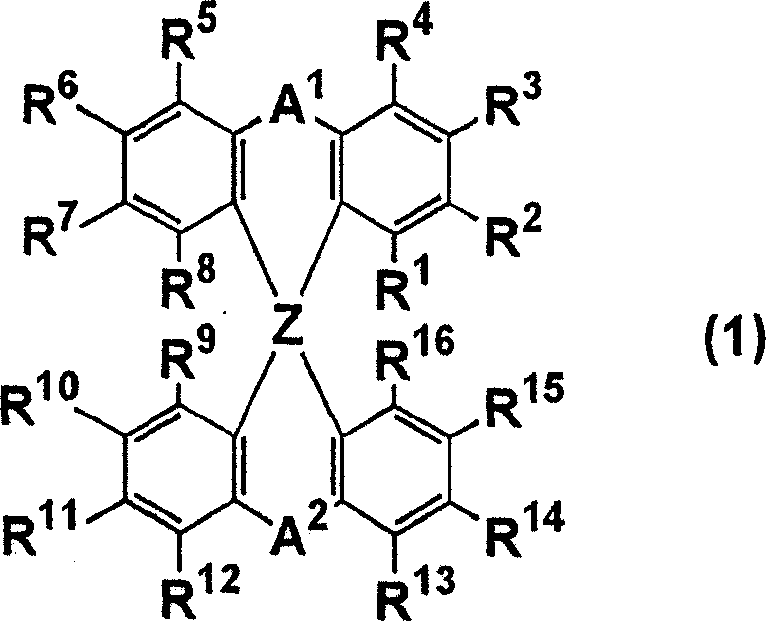
Luminescent element material and luminescent element comprising the same
A light-emitting component and component technology, which is applied in the direction of luminescent materials, electrical components, electric solid devices, etc., can solve the problems of poor color purity, cloudy film, and shortened component life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0125] Embodiment 1 (synthesis of linking group-1)
[0126]Using 2.2 g of metal magnesium, 14.8 g of 2-bromobiphenyl was Grignardized in THF, then reacted with 12.3 g of 9-xanthone at room temperature to 50° C., and treated with a conventional method to obtain 9-(2 -biphenyl)-9-xanthenol. This was heated and dehydrated in acetic acid to which a small amount of hydrochloric acid was added, and treated by a conventional method to obtain Linker-1 (8.5 g) represented by the following formula. 1 H-NMR (CDCl 3 , ppm): 7.8(d, 2H), 7.2(t, 2H), 7.2(m, 8H), 6.8(t, 2H), 6.4(d, 2H)
[0127] linker-1
Embodiment 2
[0128] Embodiment 2 (synthesis of linking group-2)
[0129] Using 1.7 g of metal magnesium, 11.9 g of 2-bromobiphenyl was Grignardized in THF, and then reacted with 13.4 g of 2,4-diethyl-9-thioxanthone at room temperature to 50°C. Processed to obtain 2,4-diethyl-9-(2-biphenyl)-9-thioxanthol. It was heated and dehydrated in acetic acid to which a small amount of hydrochloric acid was added, and treated by a conventional method to obtain Linker-2 (13.8 g) represented by the following formula. 1H-NMR (CDCl 3 , ppm): 7.8(m, 2H), 7.6(d, 2H), 7.4(m, 3H), 7.2(m, 2H), 7.1(t, 1H), 6.9(s, 1H), 6.8(t, 1H), 6.5(d, 1H), 6.2(s, 1H), 2.9(m, 2H), 2.3(m, 2H), 1.4(t, 3H), 0.9(t, 3H)
[0130] linker-2
Embodiment 3
[0131] Example 3 (Introduction of Acetyl Group to Linker-1: Linker-1')
[0132] Make the linking group-1 (8.5g) react with acetyl chloride 4.5g and aluminum chloride 7.5g in 1,2-dichloroethane at 50°C, and treat it with conventional methods to obtain the linking group shown in the following formula -1' (13.1 g). 1H-NMR (CDCl 3 , ppm): 8.0 (d, 1H), 7.9 (d, 2H), 7.8 (d, 1H), 7.7 (s, 1H), 7.4 (t, 1H), 7.3-7.1 (m, 5H), 7.0 ( s, 1H), 6.8(t, 1H), 6.3(d, 1H), 2.5(s, 3H), 2.3(s, 3H)
[0133] linker-1
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| crystallization temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com