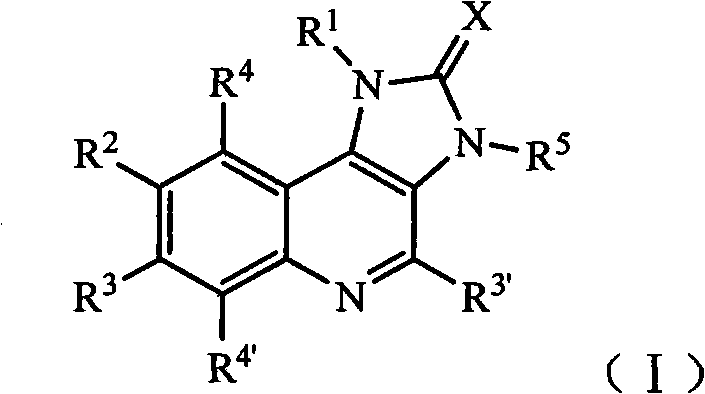
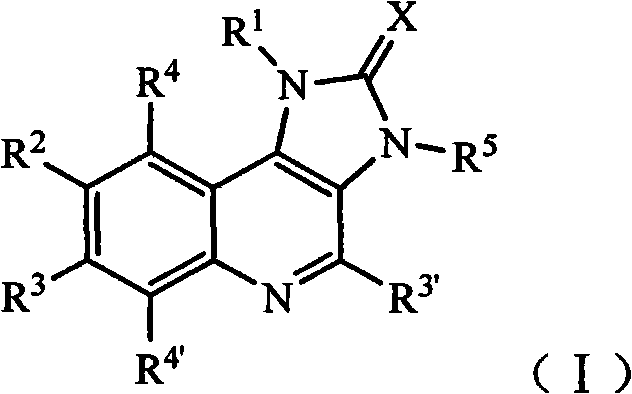
Imidazo quinoline PI3K and mTOR (mammalian target of rapamycin) dual inhibitor
A technology of CH2 and compounds, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 18
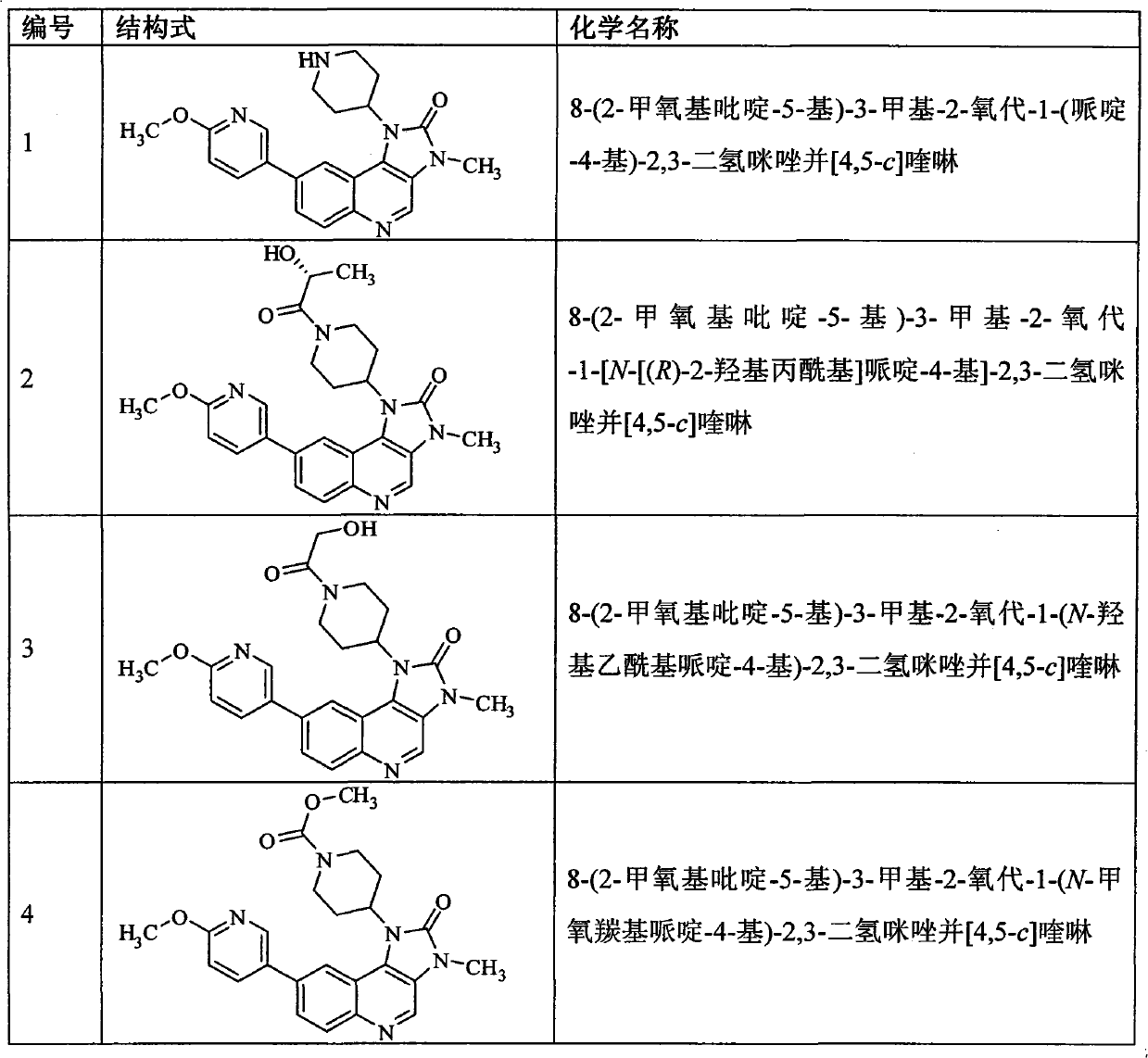
[0172] Example 18-(2-methoxypyridin-5-yl)-3-methyl-2-oxo-1-(piperidin-4-yl)-2,3-dihydroimidazo[4,5 -c] quinoline (Compound 1) Preparation
[0173] Preparation of step 1-a 2-nitro-1-hydroxyaminoethylene
[0174]
[0175] Add 6.4g (105mmol) of nitromethane into a mixture of 28g of ice and 12.4g (310mmol) of NaOH cooled in an ice bath, stir at 0°C for 1 hour, then stir at room temperature for 1 hour, add 22.4g of the solution at 0°C Ice and 32.8mL concentrated hydrochloric acid (37%) (solution A), ready to put into the next reaction immediately.
[0176] Preparation of step 1-b 5-bromo-2-(2-nitro-vinylamino)benzoic acid
[0177]
[0178] 10g (46.3mmol) of 2-amino-5-bromo-benzoic acid in H 2 Stir in a solution of O-HCl (37%) (10:1) for 2 hours, then filter to make solution B, combine solution A and solution B at room temperature, stir for 18 hours, filter out the yellow precipitate, wash with water, and dry 10.2 g of the product was obtained with a yield of 76.8%.
...
Embodiment 28
[0205] Example 28-(2-methoxypyridin-5-yl)-3-methyl-2-oxo-1-[N-[(R)-2-hydroxypropionyl]piperidin-4-yl] -2,3- Preparation of dihydroimidazo[4,5-c]quinoline (compound 2)
[0206]
[0207] Compound 1 (160mg, 0.41mmol), 1-hydroxybenzotriazole (100mg, 1.1mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (150mg, 0.8mmol), triethylamine (360mg) and D-lactic acid (72mg, 0.8mmol), the reaction solution was stirred at room temperature for 17h, the reaction solution was evaporated to dryness, and 18mg of the product was obtained through the preparative liquid phase, with a yield of 9.5%.
[0208] Molecular formula: C 25 h 27 N 5 o 4 Molecular weight: 461.51MS: 462 (M+H + )
[0209] 1 H NMR (d 6 -DMSO) δ1.19 (2H, m), 1.21 (3H, s), 2.02-2.07 (2H, m), 2.56 (1H, m), 2.87 (1H, m), 3.50 (3H, s), 3.92 (3H,s), 4.16-4.20(1H,m), 4.49-4.57(2H,m), 5.13(1H,m), 7.01(1H,d), 8.24(1H,dd), 8.72(1H,d ), 8.79(1H,d), 9.00(1H,s), 9.26(1H,d).
Embodiment 38
[0210] Example 38-(2-methoxypyridin-5-yl)-3-methyl-2-oxo-1-(N-hydroxyacetylpiperidin-4-yl)-2,3-dihydroimidium Preparation of Azolo[4,5-c]quinoline (Compound 3)
[0211]
[0212] Compound 1 (130mg 0.33mmol), 1-hydroxybenzotriazole (100mg, 1.1mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (120mg, 0.63 mmol), triethylamine (350mg) and glycolic acid (50mg, 0.66mmol), the reaction solution was stirred at room temperature for 48h, the reaction solution was evaporated to dryness, and 18mg of the product was obtained through the preparative liquid phase, with a yield of 12.3%.
[0213] Molecular formula: C 24 h 25 N 5 o 4 Molecular weight: 447.49MS: 448 (M+H + )
[0214] 1 H NMR (CDC1 3 )δ8.73(s, 1H), 8.46(d, 1H), 8.25(s, 1H), 8.24(s, 1H), 7.86(dd, 1H), 7.79-7.82(m, 1H), 6.92(d , 1H), 5.10(s, 1H), 4.93-4.95(m, 1H), 4.22-4.30(m, 2H), 4.02(s, 3H), 3.79-3.83(m, 1H), 3.60(s, 3H ), 3.20-3.26(m, 1H), 2.76-2.92(m, 3H), 2.10(d, 2H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com