Antitumor prodrug and preparation method thereof
An anti-tumor drug and anti-tumor technology, applied in the direction of anti-tumor drugs, preparation of sugar derivatives, chemical instruments and methods, etc., can solve the problems of limited application, short cycle time, poor biocompatibility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
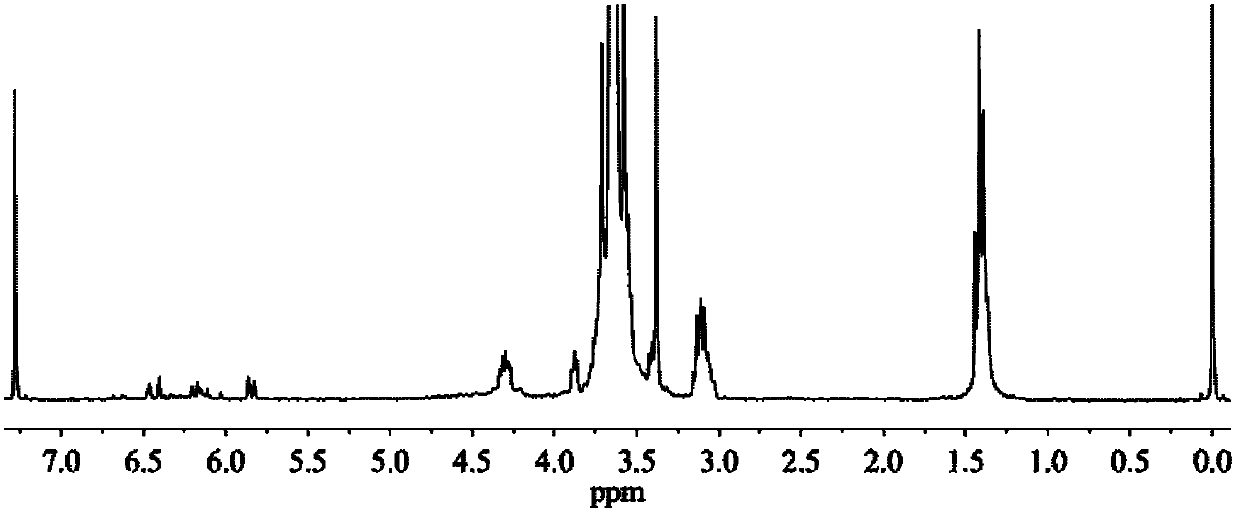
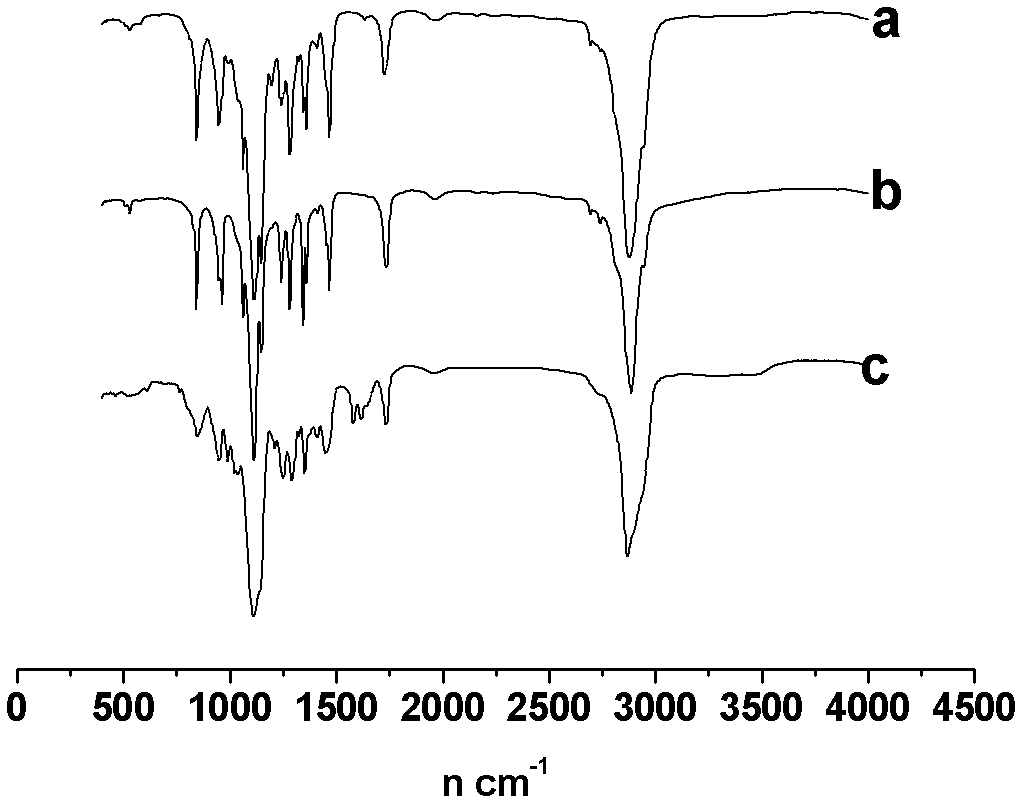
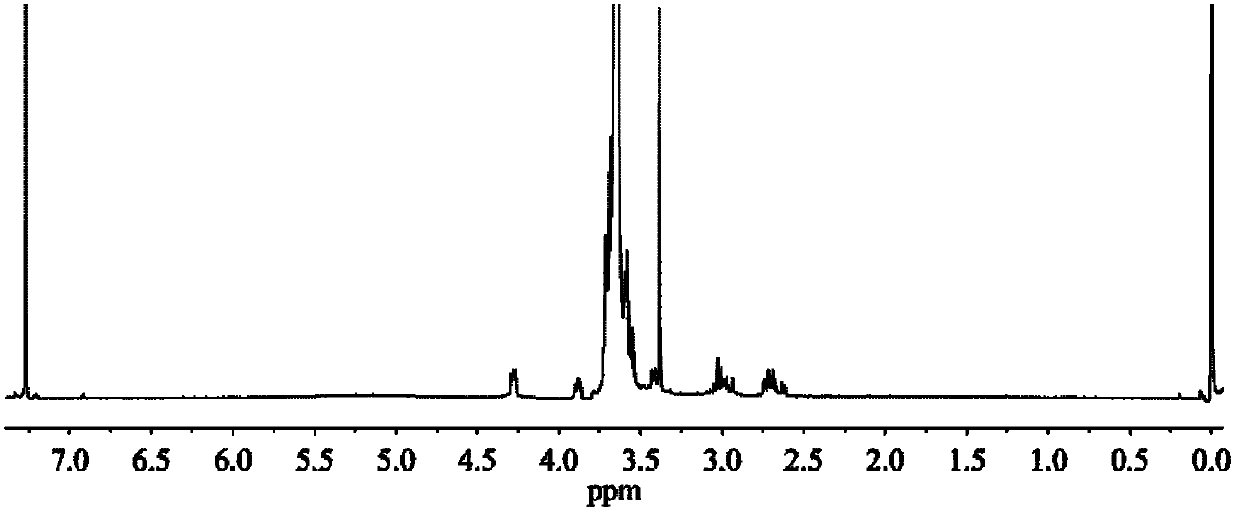
Image
Examples
preparation example Construction
[0095] The present invention also provides a preparation method of the antitumor prodrug described in the above technical scheme, comprising the following steps:
[0096] Anthracycline antineoplastic drugs, fluorenyl methaneoxycarbonyl succinimide and triethylamine are stirred and reacted in N, N-dimethylformamide to obtain amino-protected anthracycline antineoplastic drugs, and the anthracycline Antineoplastic drugs are doxorubicin, epirubicin or pirarubicin;
[0097] Stirring and reacting the amino-protected anthracycline antitumor drug, dicyclohexylcarbodiimide, 4-dimethylaminopyridine and polyethylene glycol derivatives in N,N-dimethylformamide to obtain The first intermediate product, the polyethylene glycol derivative has a structure shown in formula (III) or a structure shown in formula (IV):
[0098]
[0099] Among them, m is the degree of polymerization, 10≤m≤1500;
[0100] Piperidine is added to the first intermediate product to obtain an antitumor prodrug after...
Embodiment 6~10
[0157] Embodiment 6~10 have the preparation of the polyethylene glycol derivative of formula (IV) structure
[0158] Weigh 10 g of polyethylene glycol with a number average molecular weight of 1000 (0.01mol), 5000 (0.002mol), 10000 (0.001mol), 20000 (0.0005mol) and 40000 (0.00025mol) respectively, and put them into 5 dry In a reaction flask with a branch, add 100mL of toluene to azeotropically remove water, dissolve the obtained product in 100mL of anhydrous dichloromethane, cool to 0°C, add 10.12g, 2.02g, 1.01g, 0.51g and 0.25g of triethylamine, then add 36.20g, 7.24g, 3.62g, 1.81g and 0.91g of acryloyl chloride dropwise respectively. After the addition of acryloyl chloride, react at 0°C for 2h, return to 25°C, and continue to After the reaction was completed for 24 hours, the resulting precipitate was filtered off, and the filtrate was settled with ether, filtered, washed, and vacuum-dried at 25°C for 24 hours to obtain polyethylene glycol acrylate. Carry out nuclear magnet...
Embodiment 11
[0164] The preparation of the doxorubicin of embodiment 11 amino protection
[0165] Dissolve 200 mg of doxorubicin hydrochloride in 5 mL of N,N-dimethylformamide, add 139.6 mg of fluorenylmethoxycarbonyl succinimide and 3.5 mg of triethylamine, stir at room temperature for 3 h, and remove the solvent under reduced pressure , the residue was recrystallized with an aqueous solution of trifluoroacetic acid with a mass concentration of 0.1%, and the obtained crystals were rinsed and filtered with cold ether to remove traces of fluorenylmethoxycarbonyl succinimide, and amino-protected doxorubicin was obtained after drying.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com