Double-targeting polypeptide-antibody-drug conjugate, and prepared method and antineoplastic application thereof
A technology of drug conjugates and targeting peptides, used in antitumor drugs, drug combinations, pharmaceutical formulations, etc., can solve the problems of low drug accumulation, non-specific drug uptake by tumor cells, etc., to achieve no toxic side effects, good tumors Inhibitory effect, the effect of overcoming drug resistance and killing tumor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
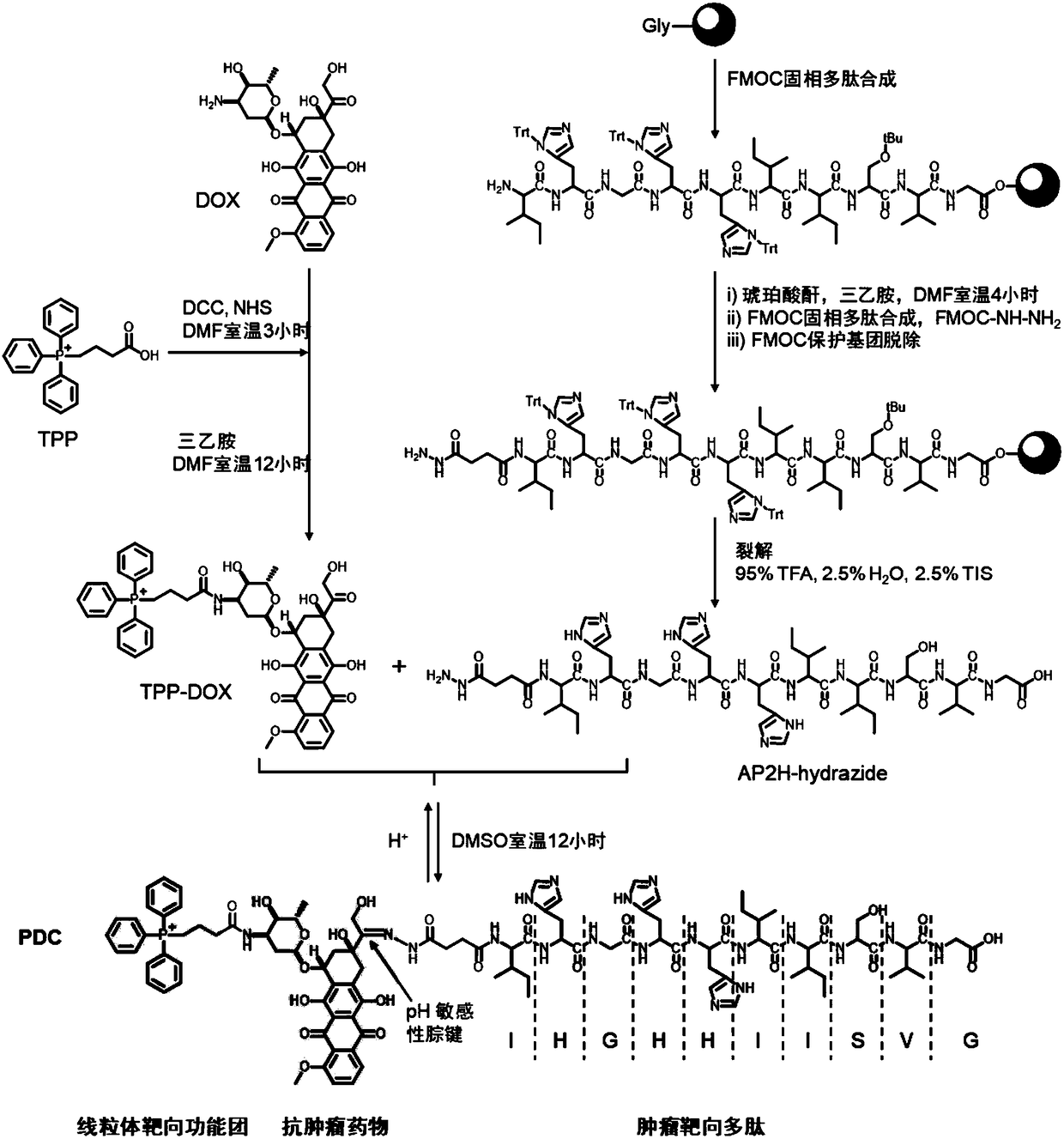
[0074] Example 1, Synthesis of Dual Targeting Polypeptide-Drug Conjugate PDC
[0075] according to figure 1 The reaction scheme for the preparation.
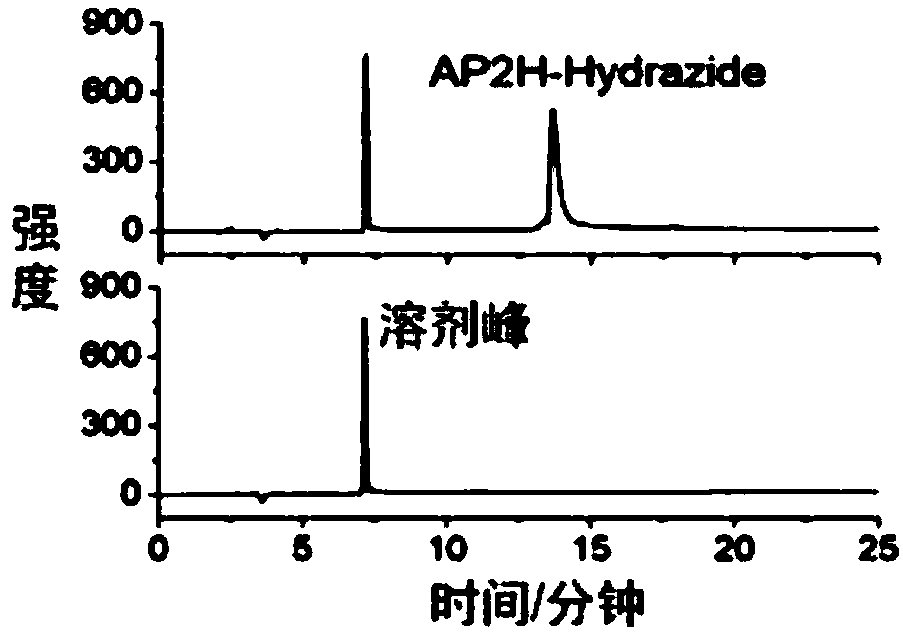
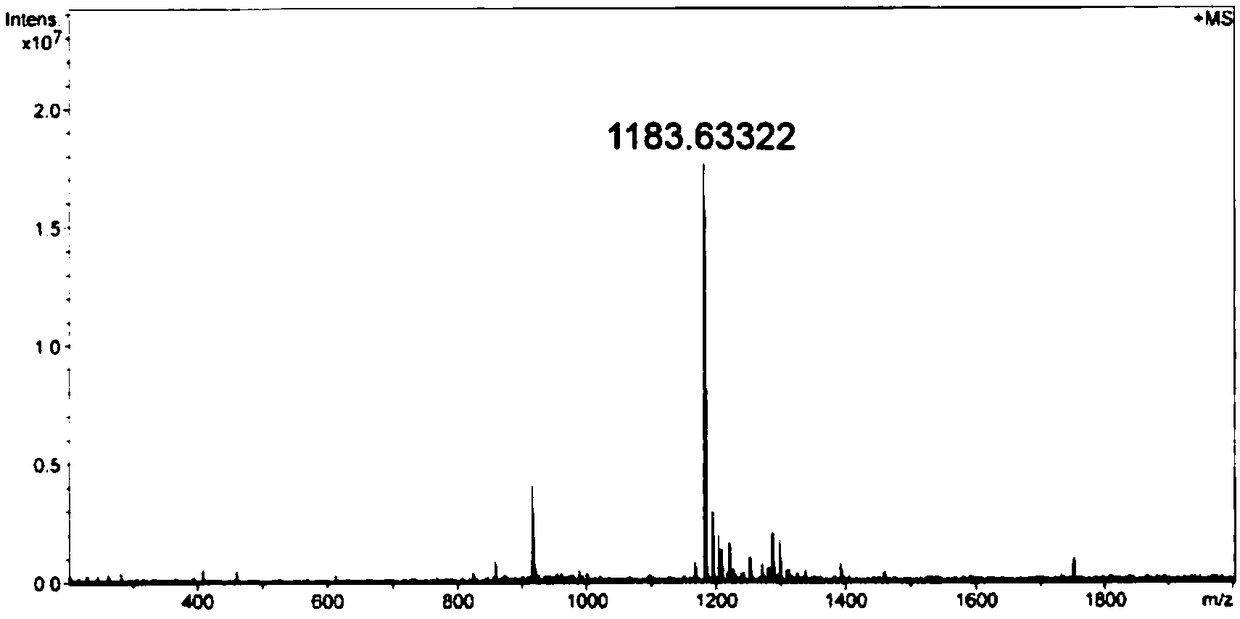
[0076] Synthesis of AP2H-hydrazide
[0077]
[0078] Synthesized manually using FMOC solid-phase synthesis strategy. FMOC-Gly-Wang resin (0.698mmol / g glycine bonded amount) was used as starting material, 20% hexahydropyridine / DMF was used as deprotection reagent, and N-methylmorpholine and HBTU were used as activation reagent.
[0079] The specific synthesis steps of the AP2H polypeptide sequence are as follows: put the weighed FMOC-Gly-Wang resin into a sand core funnel, swell with DMF for 30 minutes, and then wash with DMF three times. Add 20% hexahydropyridine / DMF solution at a ratio of 30mL / g resin, stir for 5 minutes with a magnet and then drain, repeat twice to remove the FMOC protecting group. The resin was washed six times with DMF, agitated for 1 min each, and vacuumed for 10 s. Dissolve the mixture of FMOC-AA-O...
Embodiment 2
[0085] Example 2. Performance investigation of dual-targeting polypeptide-drug conjugate PDC
[0086] 1. Fluorescence detection
[0087] DOX, TPP-DOX and PDC were respectively dissolved in DMSO to make a mother solution with a concentration of 2mM. Take 10μL of the mother solution and dilute it to 1mL with PBS, and perform fluorescence spectrum detection on a Hitachi F-4600 fluorescence instrument. The excitation wavelength is 498nm, and the record is 510-900nm range of fluorescence emission spectra.
[0088] 2. pH-sensitive drug release kinetics
[0089] In order to investigate the pH-sensitive drug release process of PDC in the solution state, the PDC mother solution was diluted to 10 μM with three different pH PBS of pH 7.4, pH 5.0 and pH 6.0, and incubated at 37°C in the dark, at different times Points (0,1,2,3,4,9,12,24,36 and 48h) to take out 100μL solution, using liquid chromatography to monitor the drug release process.
[0090] Phosphate buffered salt with pH 7.4 w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com