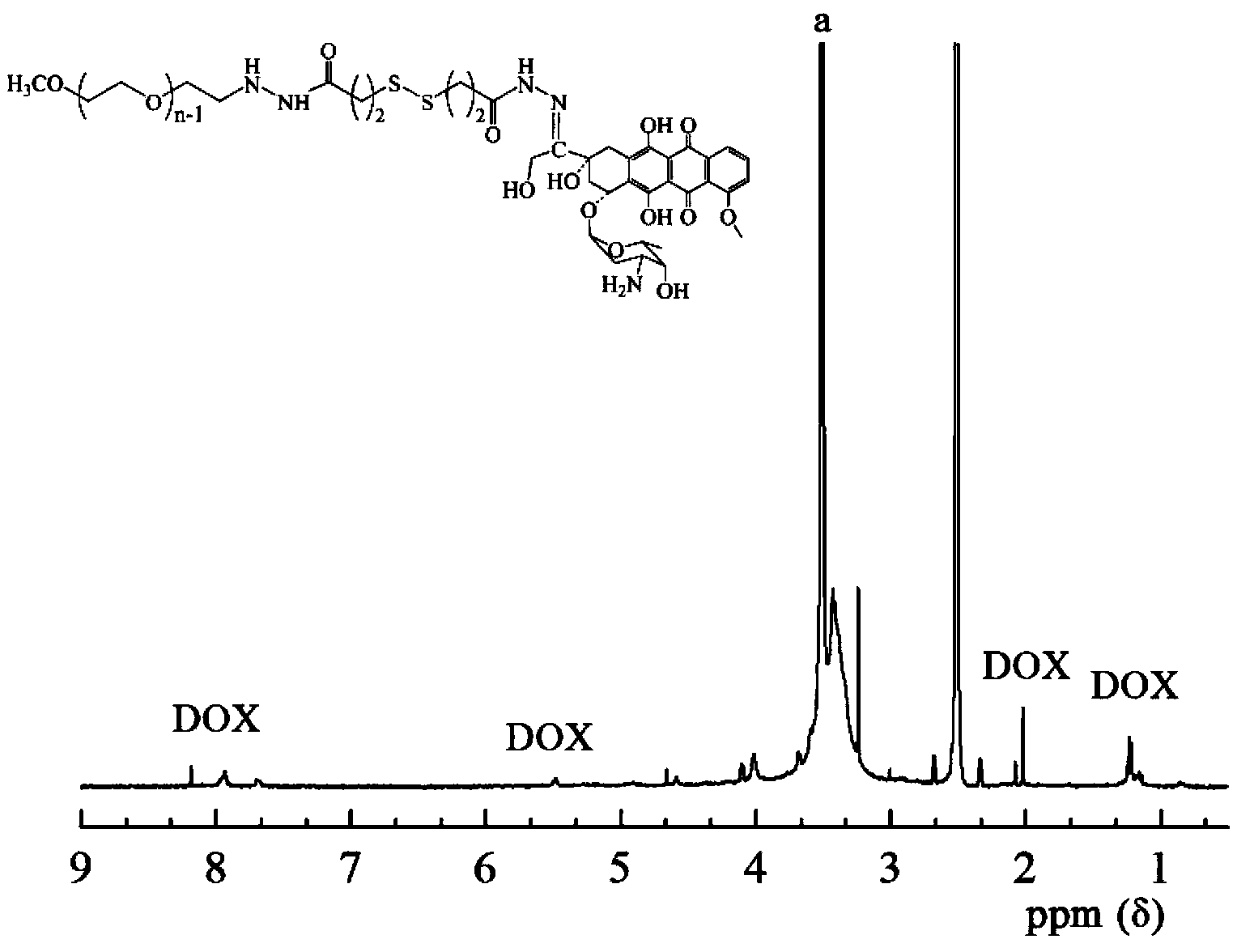
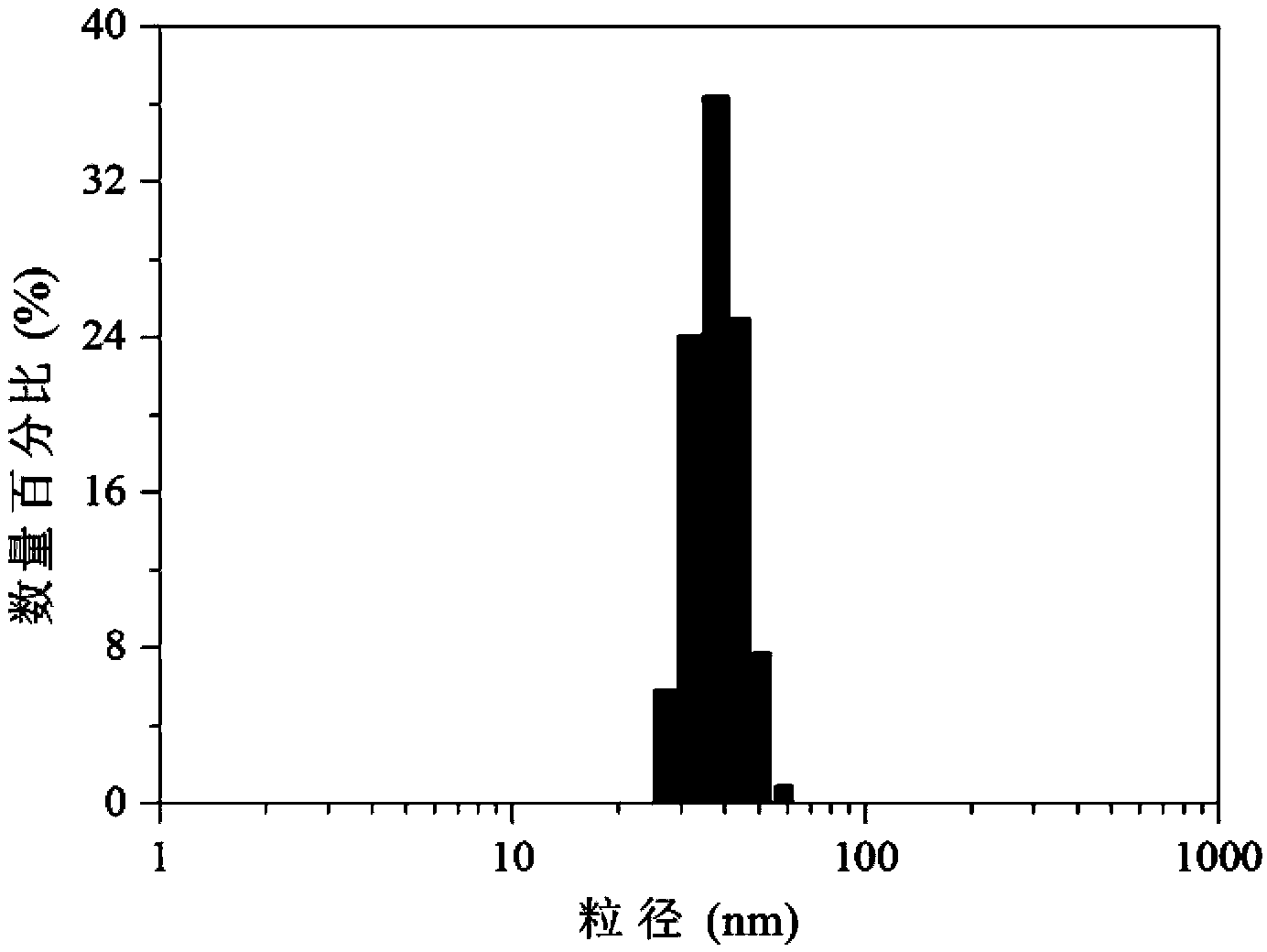
Bifunctional polyethylene glycol and adriamycin conjugate and preparation method thereof
A technology of polyethylene glycol and aldehyde-terminated polyethylene glycol, which is applied in the field of biomedicine, can solve the problems of large uncertainty in enzyme degradation and difficult control of drug release speed, so as to overcome drug resistance and reduce renal clearance rate , high curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 1) Synthesis of aldehyde-terminated polyethylene glycol:
[0046] The methoxypolyethylene glycol that molecular weight is 2000 Daltons is dissolved in the mixed solvent of anhydrous dimethyl sulfoxide and anhydrous chloroform, is mixed with the methoxypolyethylene glycol solution that mass concentration is 10%, Then within 1 hour, dropwise add acetic anhydride in the amount of 5 times the number of moles of hydroxyl groups in methoxypolyethylene glycol to the methoxypolyethylene glycol solution, stir at room temperature for 12 hours, and use the reaction solution obtained after vacuum concentration Cold ether precipitation at 20°C to obtain aldehyde-terminated polyethylene glycol; wherein, the volume ratio of anhydrous dimethyl sulfoxide and anhydrous chloroform in the mixed solvent is 75:25;
[0047] 2) Synthesis of 3,3'-dithiodipropionylhydrazide:
[0048] Add 3,3'-dithiodipropionic acid and ethanol to the reactor containing toluene and p-toluenesulfonic acid, reflux...
Embodiment 2
[0055] 1) Synthesis of aldehyde-terminated polyethylene glycol:
[0056] The methoxypolyethylene glycol that molecular weight is 1500 daltons is dissolved in the mixed solvent of anhydrous dimethyl sulfoxide and anhydrous chloroform, is formulated as the methoxypolyethylene glycol solution that mass concentration is 1%, Then within 2 hours, dropwise add acetic anhydride of 2 times the number of moles of hydroxyl groups in methoxypolyethylene glycol to the methoxypolyethylene glycol solution, and stir at room temperature for 24 hours. Cold ether precipitation at 20°C to obtain aldehyde-terminated polyethylene glycol; wherein, the volume ratio of anhydrous dimethyl sulfoxide and anhydrous chloroform in the mixed solvent is 95:5;
[0057] 2) Synthesis of 2,2'-dithiodiacetylhydrazide:
[0058] Add 2,2'-dithiodiacetic acid and methanol into a reactor filled with toluene and p-toluenesulfonic acid, reflux for 12 hours, then remove the solvent in a vacuum, and then add a mass concen...
Embodiment 3
[0065] 1) Synthesis of aldehyde-terminated polyethylene glycol:
[0066] The methoxypolyethylene glycol that molecular weight is 1200 daltons is dissolved in the mixed solvent of anhydrous dimethyl sulfoxide and anhydrous chloroform, is mixed with the methoxypolyethylene glycol solution that mass concentration is 3%, Then within 0.5 hours, dropwise add acetic anhydride of 2 times the number of moles of hydroxyl groups in methoxypolyethylene glycol to the methoxypolyethylene glycol solution, stir at room temperature for 8 hours, and use subzero Cold ether precipitation at 20°C to obtain aldehyde-terminated polyethylene glycol; wherein, the volume ratio of anhydrous dimethyl sulfoxide and anhydrous chloroform in the mixed solvent is 8:1;
[0067] 2) Synthesis of 4,4'-dithiodibutyrhydrazide:
[0068] Add 4,4'-dithiodibutyric acid and methanol into a reactor containing toluene and p-toluenesulfonic acid, reflux for 12 hours, then remove the solvent in a vacuum, and then add mass ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com