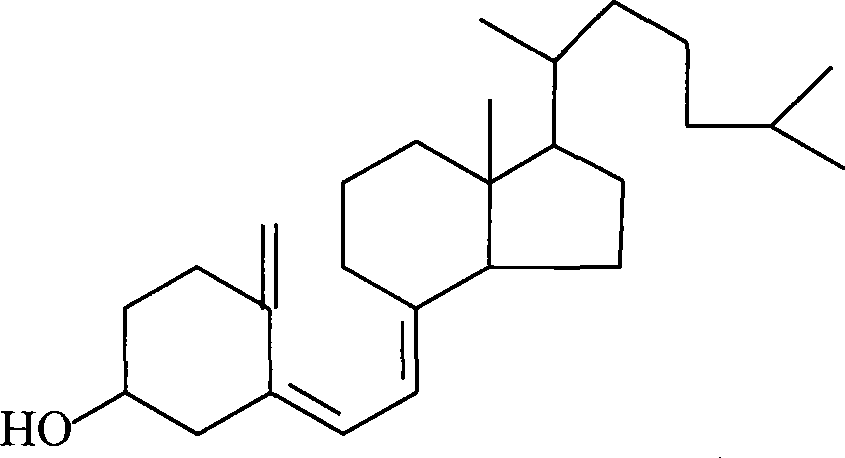
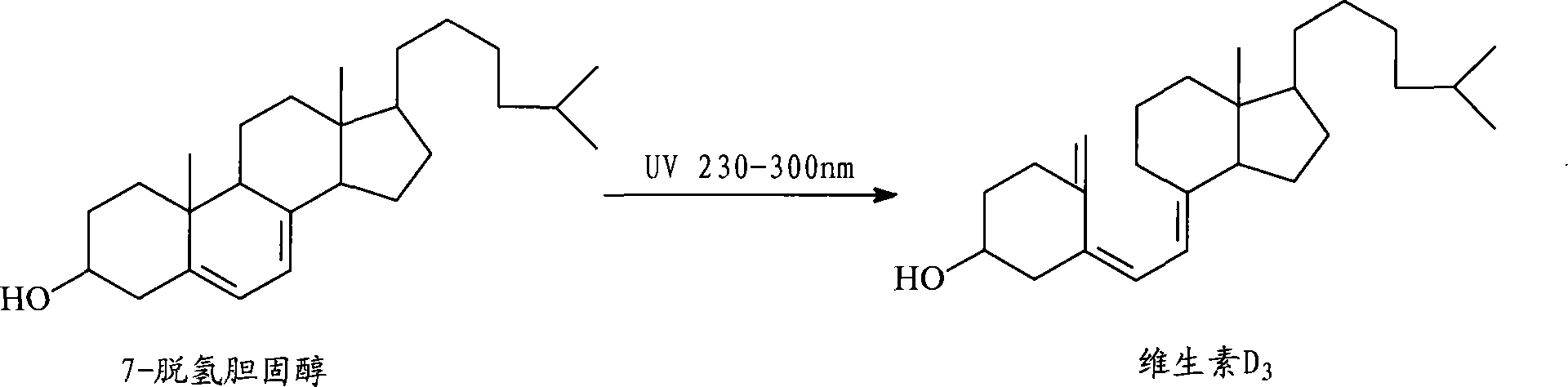
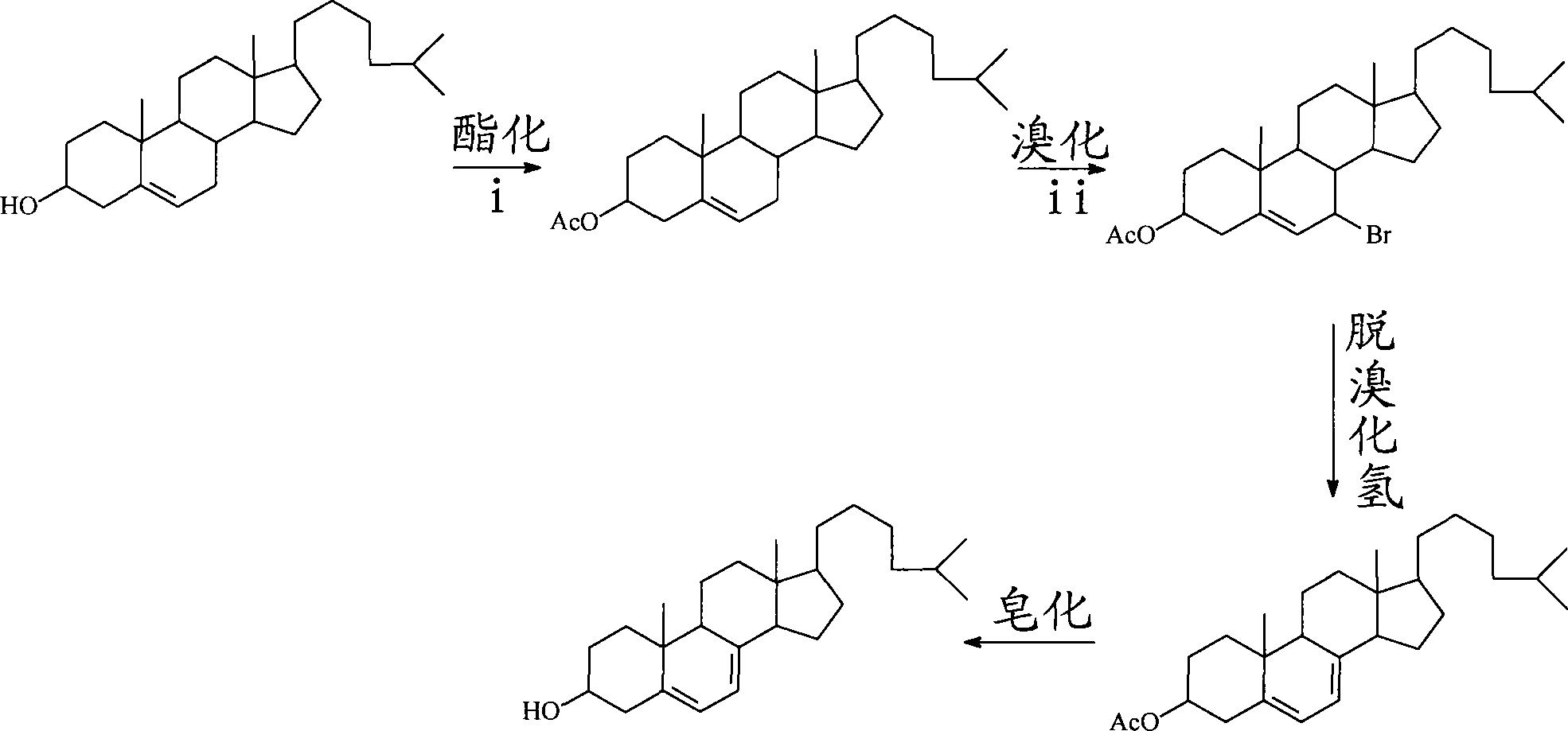
Preparation method for 7-dehydrochol esterol
A technology for dehydrocholesterol and cholesterol, applied in the field of preparation of 7-dehydrocholesterol, can solve the problems of harmful bromine environment, difficult to remove, difficult to generate conjugated diene structure and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] a) Take 5g (grams) of cholesterol and add it to 36.8ml (milliliters) of acetic anhydride, add two zeolites and reflux at 140°C±5°C for 3h to generate cholest-5ene-3-acetate. The acetic anhydride is analytically pure and has a density of 540g / 500ml.
[0059] The purity of the cholest-5-ene-3-acetate formed by the reaction was determined by the internal standard method, and the yield was 87%.
[0060] b) Dissolve 5 g of cholest-5-ene-3-acetate and 4 equivalents of anhydrous sodium acetate in 40 ml of glacial acetic acid, shake well at 25°C, and then heat to 59°C±1°C in a water bath. Take 2 equivalents of CrO 3 (Chromium trioxide) was added to the reaction solution in stages, first less and then more, first slow and then fast, and the addition was completed within 30 minutes (minutes). Stir at 59°C±1°C for 4.5h, cool to 25°C, then cool with water at 4°C for 12h, crystals will precipitate, use a glass sand core funnel to filter, and then wash the crystals with deionized w...
Embodiment 2
[0068] a) Dissolve 5 g of cholesterol in 40 ml of dried benzene, then add 2 equivalents of acetic anhydride, reflux at 80° C. for 3 h. After the reaction solution is cooled to 25°C, wash it with 1% dilute hydrochloric acid to weak acidity and then use 5% NaHCO 3 (sodium bicarbonate) aqueous solution to weak alkaline, and finally to neutral with deionized water. The aqueous phase was extracted 3 times with an appropriate amount of ether, and the benzene layers after the three extractions were combined and washed with anhydrous Na 2 SO 4 dry. The dried benzene-ether solution was rotary evaporated to obtain 5.44 g of cholest-5-ene-3-acetate.
[0069] The tested purity is 97%, and the yield of cholest-5-ene-3-acetate is greater than 95%.
[0070] b) Dissolve 4ml of tert-butanol peroxide with a volume fraction of 70% and 1.71g (4mmol) of cholest-5-ene-3-acetate in 16ml of dichloromethane, and stir at 25°C for 24h, Then the solvent was rotary evaporated to obtain 2.61 g of soli...
Embodiment 3
[0074] In this embodiment, step b) is different from Embodiment 1, and other steps are the same as those in Embodiment 1. Step b) of the present embodiment is:
[0075] Take 2.0 grams of cholesterol acetate and 0.029 equivalent of cuprous iodide, dissolve it in 30 ml of benzene, and finally add 5.6 equivalents of tert-butyl hydroperoxide. Under the protection of nitrogen, the reaction was stirred at 70°C for 26h.
[0076] The obtained cholest-5-ene-7-carbonyl-3-acetate solid was checked for purity and the calculated yield was 30%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com