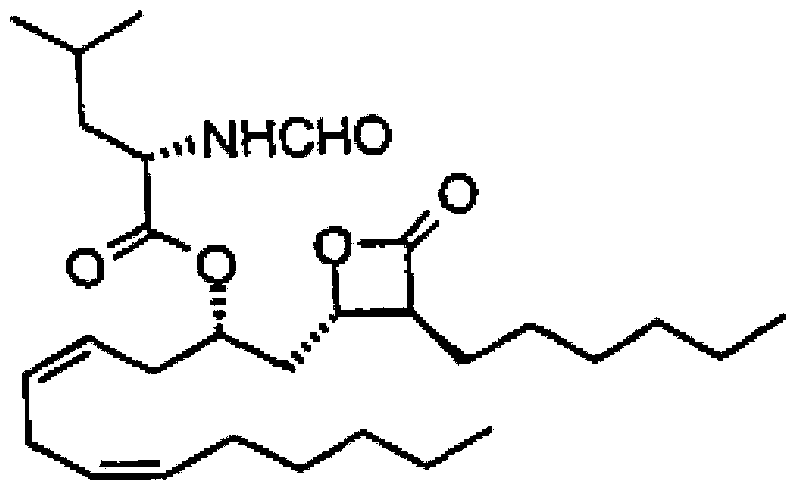
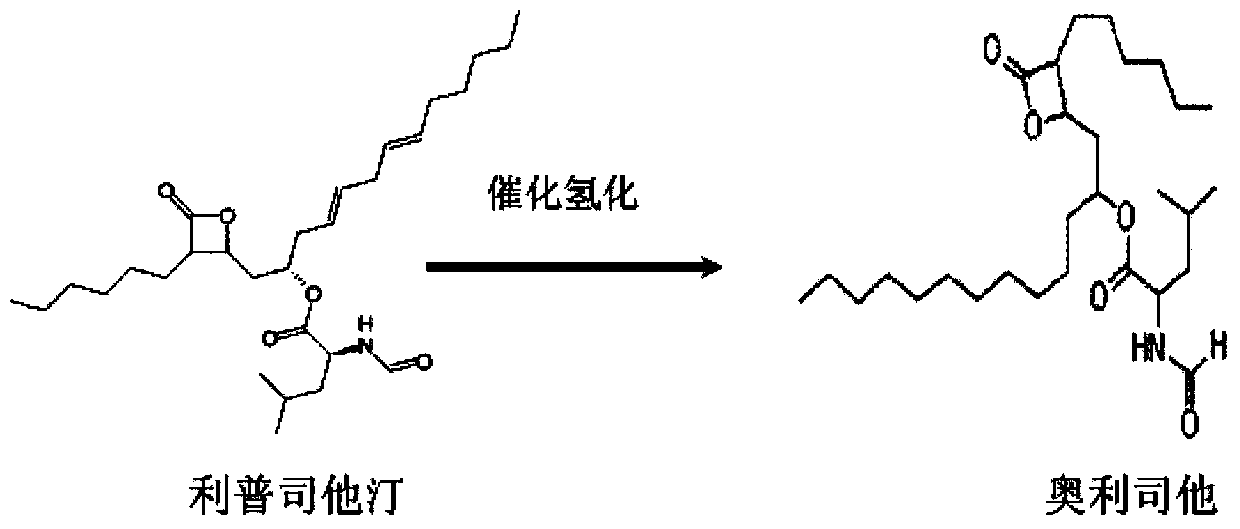
Method for purifying orlistat intermediate
A statin, medium technology, applied in the field of medicine, can solve the problems of high equipment requirements, long production cycle, high production cost, etc., and achieve the effect of less capital required for equipment, low equipment requirements, and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
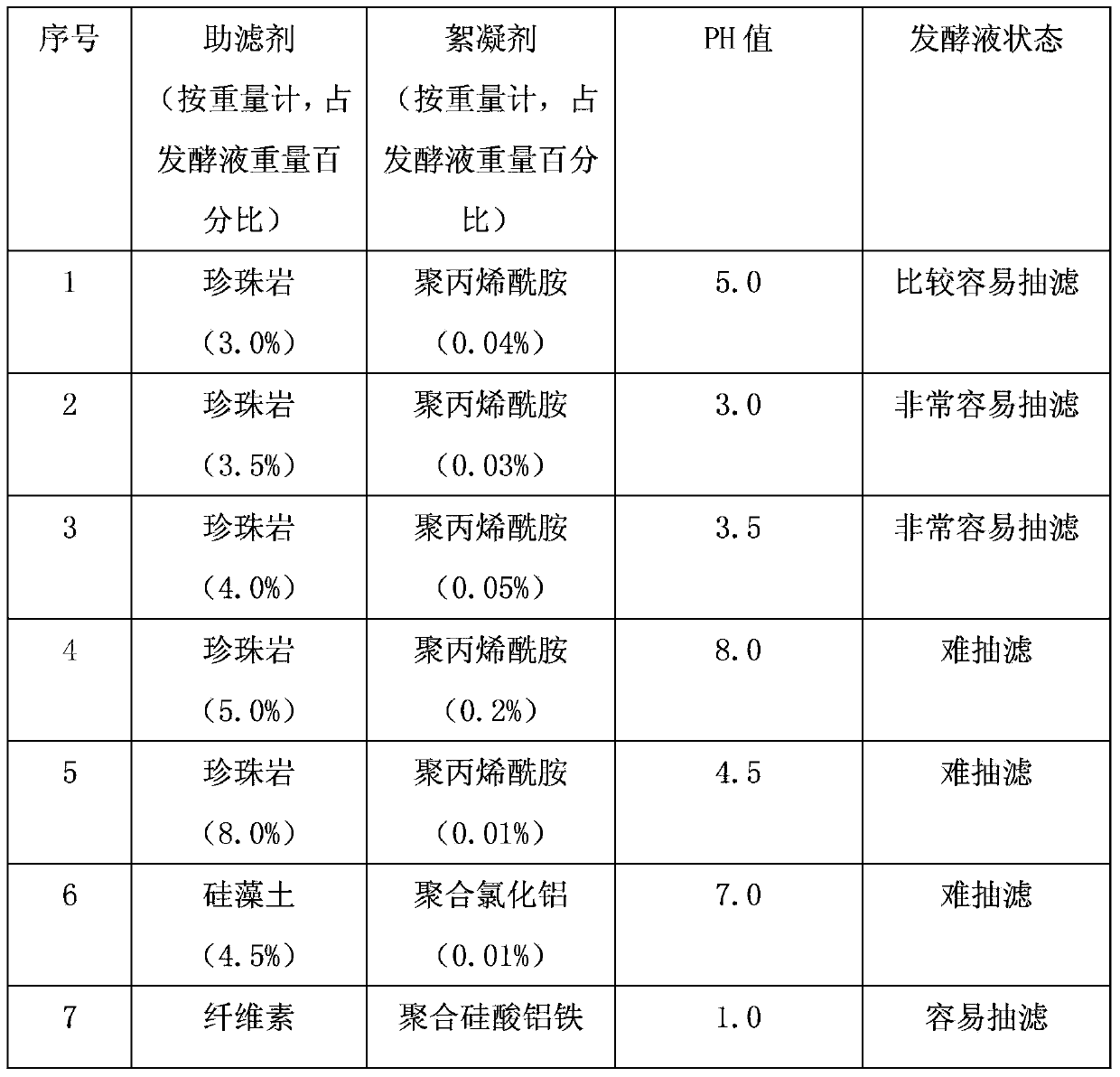
[0052] 1) Fermentation broth pretreatment: take 5t of freshly cultivated liprestatin fermentation broth, add 150kg of filter aid magnesium oxide, 2.8kg of flocculant ferric chloride, adjust the pH to 4.0 with hydrochloric acid, and filter with plate and frame to obtain 1t containing A mixture of liprestatins.
[0053] 2) Extraction: Add the liprestatin-containing mixture into 6000L isopropanol solution for microbial cell disruption, stir for 1 hour, and plate-frame filter to obtain an extract containing liprestatin.
[0054] 3) Phase inversion:
[0055] Phase inversion for the first time: add 3600L of drinking water and 3600L of petroleum ether to the leaching solution, stir for 10 minutes, then let stand for 1 hour to separate layers, and take the upper petroleum ether phase;
[0056] The second phase inversion: add 2160L isopropanol to the petroleum ether phase, stir for 5 minutes and let it stand for 1 hour to separate the layers. Use TLC to spot the isopropanol phase to j...
Embodiment 2
[0059] 1) Fermentation broth pretreatment: Take 5t of freshly cultivated liprestatin fermentation broth, add 200kg of filter aid perlite, 500ml of flocculant polyacrylamide, adjust the pH to 3.5 with hydrochloric acid, and filter with plate and frame to obtain 1t of liprestatin containing A mixture of statins.
[0060] 2) Extraction: add the liprestatin-containing mixture into 4000L ethanol solution for microbial cell disruption, stir for 1 hour, and plate-frame filter to obtain the liprestatin-containing mixture extract.
[0061] 3) Phase inversion:
[0062] Phase inversion for the first time: add 2000L drinking water and 2000L heptane to the extract solution, stir for 10 minutes, then let stand for 1 hour to separate layers, and take the upper heptane phase;
[0063] The second phase inversion: add 1000L acetonitrile to the heptane phase, stir for 5 minutes and let it stand for 1 hour to separate the layers. Use TLC to spot the acetonitrile phase to judge the effect of remo...
Embodiment 3
[0066] 1) Fermentation broth pretreatment: Take 5t of freshly cultivated liprestatin fermentation broth, add 200kg of filter aid cellulose, 0.5kg of flocculant polymerized aluminum iron silicate, adjust the pH to 6.0 with hydrochloric acid, and filter with plate and frame to obtain 1t A mixture containing liprestatin.
[0067] 2) Extraction: Add the mixture containing riprestatin to 4000L isopropanol solution for microbial cell disruption, stir for 1 hour, and filter with a plate frame to obtain an extract of the mixture containing riprestatin.
[0068] 3) Phase inversion:
[0069] Phase inversion for the first time: add 2400L drinking water and 2000L n-hexane to the extract solution, stir for 10 minutes, then let stand for 1 hour to separate layers, and take the upper n-hexane phase;
[0070] Wash with water to remove impurities: add 600L of drinking water to the n-hexane phase, stir for 5 minutes, let stand for 1 hour to separate layers, and take the upper n-hexane phase; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com