A method for preparing weight-loss drug orlistat
A technology of orlistat and statin, which is applied in the field of preparation of weight-loss drug orlistat, can solve the problems of harsh hydrogenation reaction conditions and low yield, and achieve high reaction yield, less reaction by-products, and simple post-treatment Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
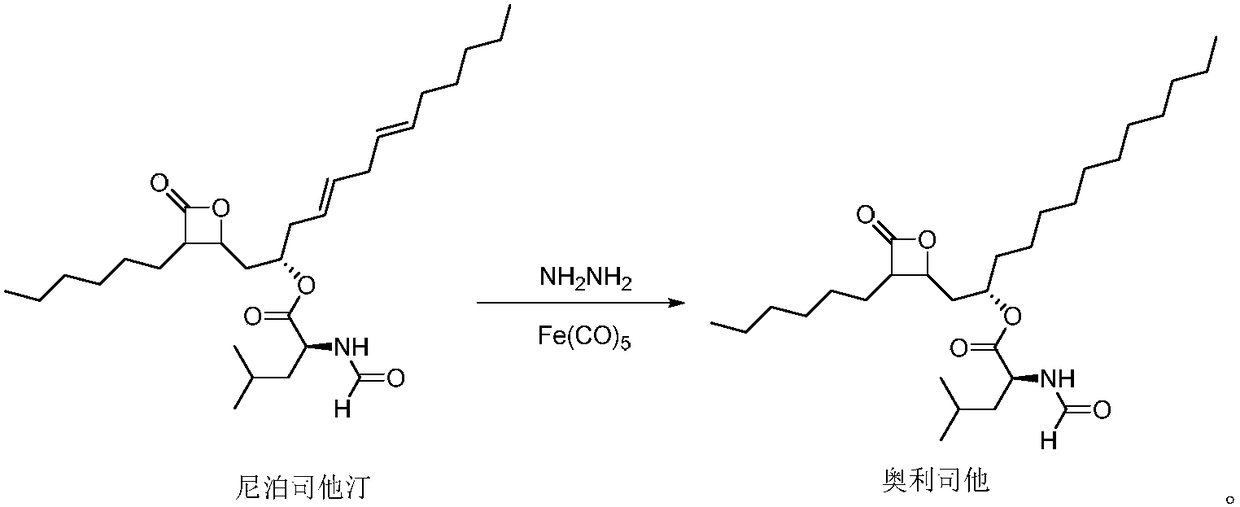
[0020] Preparation of orlistat
[0021] First, the reaction flask was replaced with nitrogen three times, then 10 g of niborestatin and 0.11 g of iron pentacarbonyl were added to tetrahydrofuran at room temperature, and then heated to 50°C, keeping the temperature, and 3.1 g of hydrazine hydrate was added dropwise. React for 5 hours, cool to room temperature, filter, and concentrate the filtrate under reduced pressure to obtain orlistat. Dissolve the obtained orlistat in a mixed solvent of dichloromethane / petroleum ether (volume ratio 1:20), then heat to 65°C and stir for 10-20 minutes, filter while hot, cool the filtrate naturally, suction filter, and vacuum-dry to obtain refined 9.1 g of orlistat, the yield was 90.2%, and the HPLC purity was 99.76%.
Embodiment 2
[0023] Preparation of orlistat
[0024] First, the reaction flask was replaced with nitrogen three times, then 10 g of niborestatin and 0.1 g of iron pentacarbonyl were added to tetrahydrofuran at room temperature, and then heated to 45 ° C, keeping the temperature, and 3.1 g of hydrazine hydrate was added dropwise. React for 6 hours, cool to room temperature, filter, and concentrate the filtrate under reduced pressure to obtain orlistat. The obtained orlistat was dissolved in dichloromethane / petroleum ether mixed solvent (volume ratio 1:20), then heated to 70°C and stirred for 10-20 minutes, filtered while hot, the filtrate was naturally cooled, suction filtered, and vacuum-dried to obtain refined 8.9 g of orlistat, the yield was 88.2%, and the HPLC purity was 99.63%.
Embodiment 3
[0026] Preparation of orlistat
[0027] First, the reaction flask was replaced with nitrogen three times, then 10 g of niborestatin and 0.2 g of iron pentacarbonyl were added to tetrahydrofuran at room temperature, and then heated to 40°C, keeping the temperature, and 4.1 g of hydrazine hydrate was added dropwise. React for 6 hours, cool to room temperature, filter, and concentrate the filtrate under reduced pressure to obtain orlistat. Dissolve the obtained orlistat in dichloromethane / petroleum ether mixed solvent (volume ratio 1:20), then heat to 80°C and stir for 10-20 minutes, filter while hot, cool the filtrate naturally, suction filter, and vacuum-dry to obtain refined 8.9 g of orlistat, the yield was 88.7%, and the HPLC purity was 99.59%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com