Production process for preparing lipstatin
A production process and statin technology, applied in the directions of microorganisms, microorganism-based methods, biochemical equipment and methods, etc., can solve the problems of low fermentation titer and high production cost, and achieve the effect of increasing fermentation titer and reducing production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
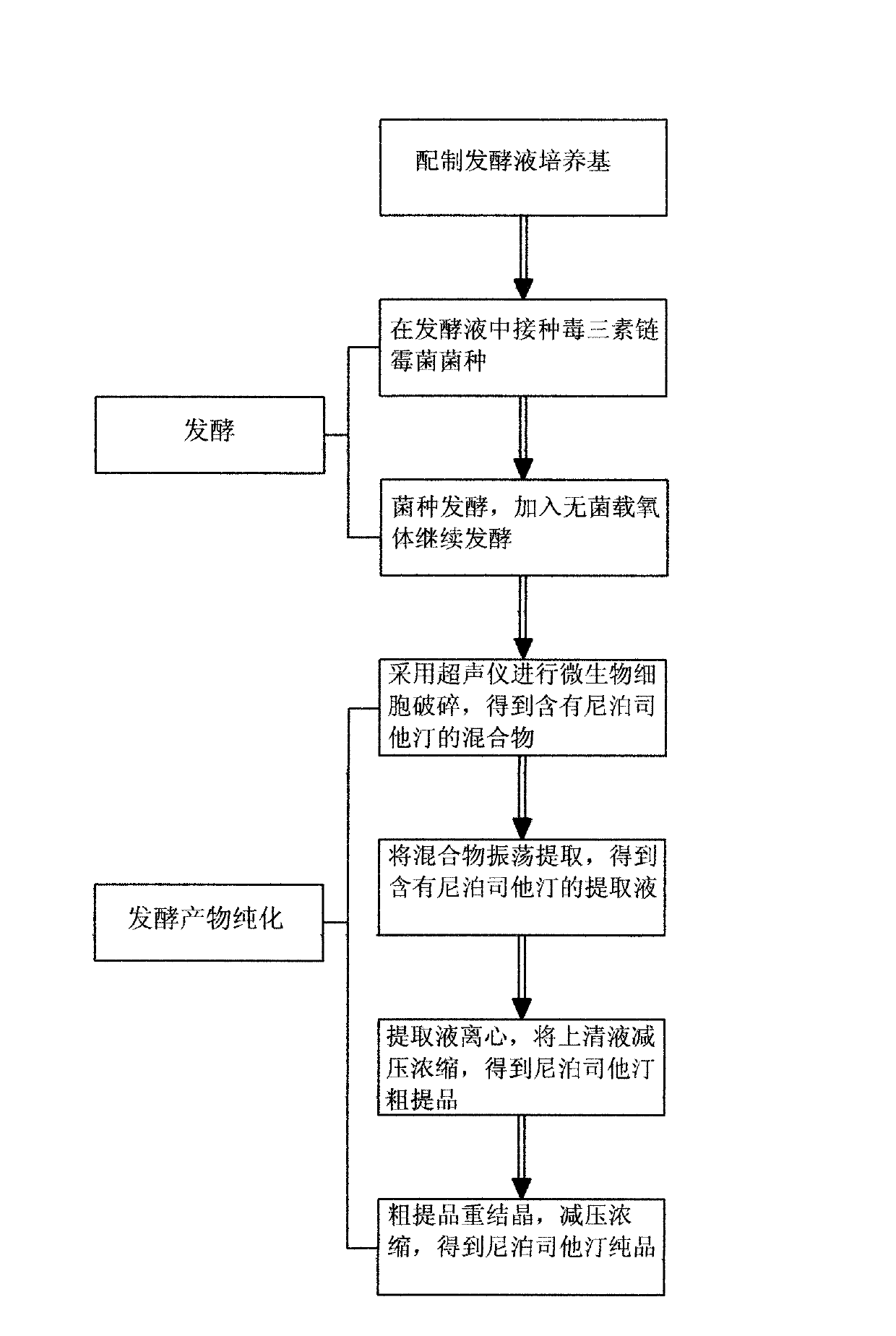
[0023] Example 1: See figure 1 , the invention discloses a production process for preparing niborestatin, comprising the following process:
[0024] A. Preparation of fermentation broth culture medium
[0025] The fermentation medium is made of glycerin, soybean powder, lecithin, soybean oil, magnesium sulfate, calcium carbonate and potassium dihydrogen phosphate, and the fermentation medium contains 4.0% of glycerin, 4.5% of soybean powder, and 1.5% of lecithin %, soybean oil 5%, magnesium sulfate 0.05%, calcium carbonate 0.05%, potassium dihydrogen phosphate 0.05%.
[0026] B. Fermentation
[0027] B1. Streptomyces toxin was inoculated in the fermentation broth, and the bacterial concentration of the strain was 15%.
[0028] B2. During the strain fermentation process, the temperature of the fermented liquid is controlled at about 25°C. When fermenting for 60-80 hours, add sterile oxygen carrier in an amount of 0.05g / L, and continue fermenting for 100-120 hours.
[0029] ...
Embodiment 2
[0035] Example 2: See figure 1 , the invention discloses a production process for preparing niborestatin, comprising the following process:
[0036] A. Preparation of fermentation broth culture medium
[0037] The fermentation medium is made of glycerin, soybean powder, lecithin, soybean oil, magnesium sulfate, calcium carbonate and potassium dihydrogen phosphate, and the fermentation medium contains 5.0% of glycerin, 6% of soybean powder, and 2.5% of lecithin %, soybean oil 8%, magnesium sulfate 0.1%, calcium carbonate 0.1%, potassium dihydrogen phosphate 0.1%.
[0038] B. Fermentation
[0039] B1. Streptomyces toxin was inoculated in the fermentation broth, and the bacterial concentration of the strain was 20%.
[0040] B2. During the strain fermentation process, the temperature of the fermentation liquid is controlled at about 25°C. When the fermentation is 60-80 hours, add a sterile oxygen carrier in an amount of 0.1g / L, and continue the fermentation for 100-120 hours. ...
Embodiment 3
[0047] Example 3: See figure 1 , the invention discloses a production process for preparing niborestatin, comprising the following process:
[0048] A. Preparation of fermentation broth culture medium
[0049] The fermentation medium is made of glycerin, soybean powder, lecithin, soybean oil, magnesium sulfate, calcium carbonate and potassium dihydrogen phosphate, and the fermentation medium contains 6.5% of glycerin, 7.5% of soybean powder, and 3.0% of lecithin %, soybean oil 10%, magnesium sulfate 0.2%, calcium carbonate 0.2%, potassium dihydrogen phosphate 0.2%.
[0050] B. Fermentation
[0051] B1. Streptomyces toxin was inoculated in the fermentation broth, and the bacterial concentration of the strain was 25%.
[0052] B2. During the strain fermentation process, the temperature of the fermentation liquid is controlled at about 25°C. When the fermentation is 60-80 hours, add sterile oxygen carrier, the amount added is 0.15g / L, and the fermentation is continued for 100-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com