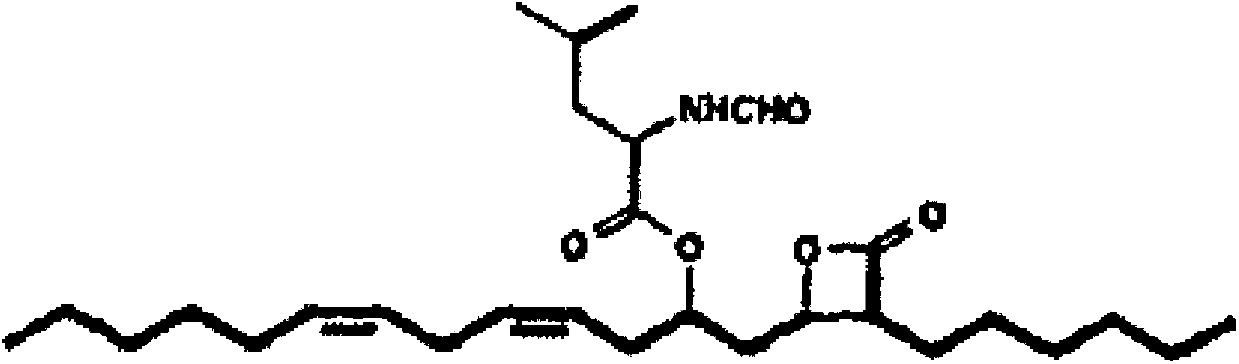
Method for purifying orlistat
A technology of orlistat and purification methods, applied in the direction of organic chemistry, etc., can solve the problems of low product content, high filler cost, and low product quality, and achieve the effects of increased production costs, reduced production costs, and high product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Orlistat crude product 1000g, product content 80% (weight / weight), maximum 0.4% simple impurities, add 20L methanol to dissolve, filter to obtain filtrate, add purified water with a volume fraction of 15% to the filtrate for methanol crystallization, 0°C Suction filtration to obtain orlistat primary refined product. According to HPLC detection, the content of orlistat is 90%, the highest is 0.2%.
[0024] Dissolve the primary refined product of methanol with petroleum ether, maintain its concentration at 40g / L, add 1 / 2 of the purified water of the system to wash and remove impurities, then add ethanol with a volume fraction of 1% to the system, crystallize at low temperature, 1 °C suction filtration to obtain the secondary refined product of orlistat. According to HPLC detection, the content of orlistat is 99.2%, the maximum single impurity is 0.092%, and the yield of two-step refining is 85%.
Embodiment 2
[0026] Orlistat crude product 1000g, product content 68% (weight / weight), maximum 0.42% simple impurities, add 40L ethanol to dissolve, filter to obtain filtrate, add purified water with a volume fraction of 20% to the filtrate for ethanol crystallization, 2°C Suction filtration to obtain orlistat primary refined product. According to HPLC detection, the content of orlistat is 92%, and the highest is 0.21%.
[0027] Dissolve the first-time refined product of ethanol in heptane, maintain its concentration at 30g / L, add 1 / 2 of the purified water of the system to wash and remove impurities, and then add methanol with a volume fraction of 0.8% to the system for low-temperature crystallization, 2 °C suction filtration to obtain the secondary refined product of orlistat. According to HPLC detection, the content of orlistat is 99.5%, the maximum single impurity is 0.086%, and the yield of two-step refining is 84%.
Embodiment 3
[0029] Orlistat crude product 1000g, product content 84% (weight / weight), maximum 0.3% impurity, add 25L acetonitrile to dissolve, filter to obtain filtrate, add purified water with a volume fraction of 17% to the filtrate for acetonitrile crystallization, 5 ℃ Suction filtration to obtain orlistat primary refined product. According to HPLC detection, the content of orlistat is 95%, and the highest is 0.15%.
[0030] After dissolving the first-time refined product of acetonitrile with hexane, keep its concentration at 40g / L, add 1 / 2 of the purified water of the system to wash and remove impurities, then add ethanol with a volume fraction of 0.2% to the system, crystallize at low temperature, 8 °C suction filtration to obtain the secondary refined product of orlistat. According to HPLC detection, the content of orlistat is 99.2%, the maximum single impurity is 0.065%, and the yield of two-step refining is 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com