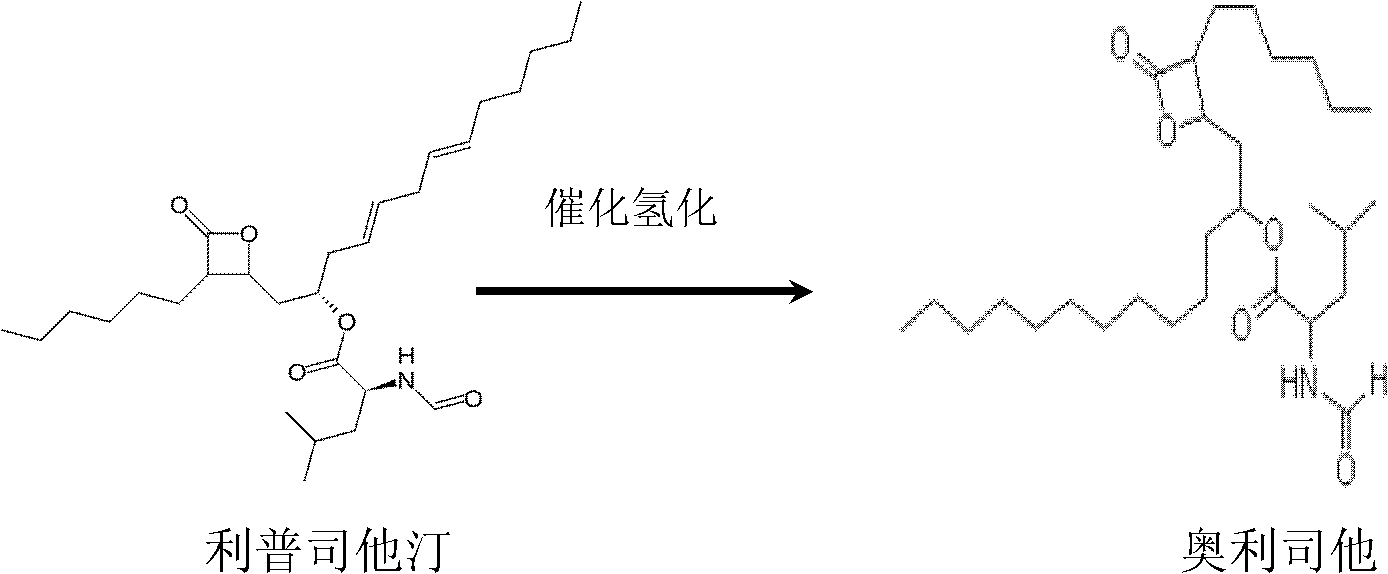
Method for preparing high-purity Orlistat
An orlistat, high-purity technology, applied in the field of medicine, can solve the problems of difficulty in scaling up, high equipment investment, and high production costs, and achieve the effects of cleaner production, less investment, and lower production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1, a kind of method for preparing high-purity orlistat
[0037] ①Extraction: 3M 3 The liprestatin fermentation broth was filtered through a plate and frame filter press, and the filter cake was blown dry with nitrogen, and the filtered bacteria were transferred to an extraction tank, and ethanol 5 times the weight of the bacteria was added, and stirred and extracted for 8 hours. Collect the filtrate by plate and frame filtration;
[0038] ②Extraction: add water to the filtrate to adjust the ethanol concentration to 40%, add 1 / 5 of heptane according to the volume of the filtrate for extraction, stir, stand and separate, collect the upper phase, concentrate the upper phase under reduced pressure to 1 / 5 of the original volume 7. Remove the insoluble particles by filtration to obtain a concentrated solution of lipostatin with a concentration of 220g / L, which is to be applied to the chromatographic column;
[0039] ③ Chromatography: Prepare a stainless steel med...
Embodiment 2
[0046] Embodiment 2, a kind of method for preparing high-purity orlistat
[0047] ①Extraction: 3M 3 The liprestatin fermentation broth was filtered through a plate and frame filter press, and the filter cake was blown dry with nitrogen, and the filtered bacteria were transferred to an extraction tank, and methanol 10 times the weight of the bacteria was added, and stirred and extracted for 4 hours. Collect the filtrate by plate and frame filtration;
[0048] ②Extraction: add water to the filtrate to adjust the concentration of methanol to 65%, add 1 / 2 of ethyl acetate according to the volume of the filtrate for extraction, stir, let stand to separate layers, collect the upper phase, and concentrate the upper phase under reduced pressure to 1% of the original volume. / 8, filter to remove insoluble particles, get the lipostatin concentrated solution, the concentration is 220g / L, and wait for the upper chromatographic column;
[0049] ③ Chromatography: Prepare a stainless steel...
Embodiment 3
[0056] Embodiment 3, a kind of method for preparing high-purity orlistat
[0057] ①Extraction: 3M 3 The liprestatin fermentation broth was filtered through a plate and frame filter press, and the filter cake was blown dry with nitrogen, and the filtered bacteria were transferred to an extraction tank, and acetone 4 times the weight of the bacteria was added, and stirred and extracted for 12 hours. Collect the filtrate by plate and frame filtration;
[0058] ②Extraction: add water to the filtrate to adjust the concentration of acetone to 30%, add 1 / 6 of petroleum ether according to the volume of the filtrate for extraction, stir, stand and separate layers, collect the upper phase, concentrate the upper phase under reduced pressure to 1 / 3 of the original volume 4. Remove the insoluble particles by filtration to obtain a concentrated solution of lipostatin with a concentration of 210g / L, which is to be applied to the chromatographic column;
[0059] ③ Chromatography: Prepare a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com