Orlistat-containing pharmaceutical composition for reducing weight
A technology of orlistat and composition, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
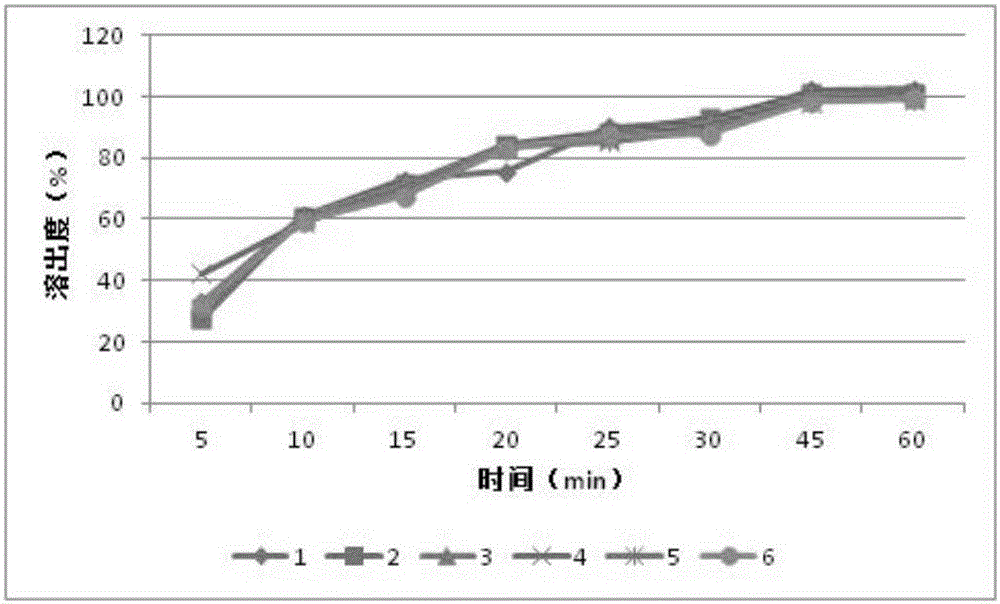
[0023] Example 1 Preparation of capsules containing orlistat and fructooligosaccharides
[0024] Prescription: (based on 1000 capsules):
[0025]
[0026] Preparation Process:
[0027] Sodium lauryl sulfate, povidone K30, sodium carboxymethyl starch, microcrystalline cellulose, orlistat, and fructooligosaccharide powder were all passed through an 80-mesh sieve before use. Take sodium lauryl sulfate, povidone K30, carboxymethyl starch sodium, microcrystalline cellulose, orlistat, fructooligosaccharide powder of prescription quantity, first a small amount of sodium lauryl sulfate, povidone K30 and carboxymethyl starch sodium were mixed evenly, then added microcrystalline cellulose, orlistat, and fructooligosaccharides, mixed evenly, and passed through an 80-mesh sieve twice. Slowly add 50% ethanol solution containing 10% povidone K30 to the mixed powder to make a soft material, extrude and sieve through a 20-mesh granule, dry the wet granule in a blast drying oven at 30°C f...
Embodiment 2
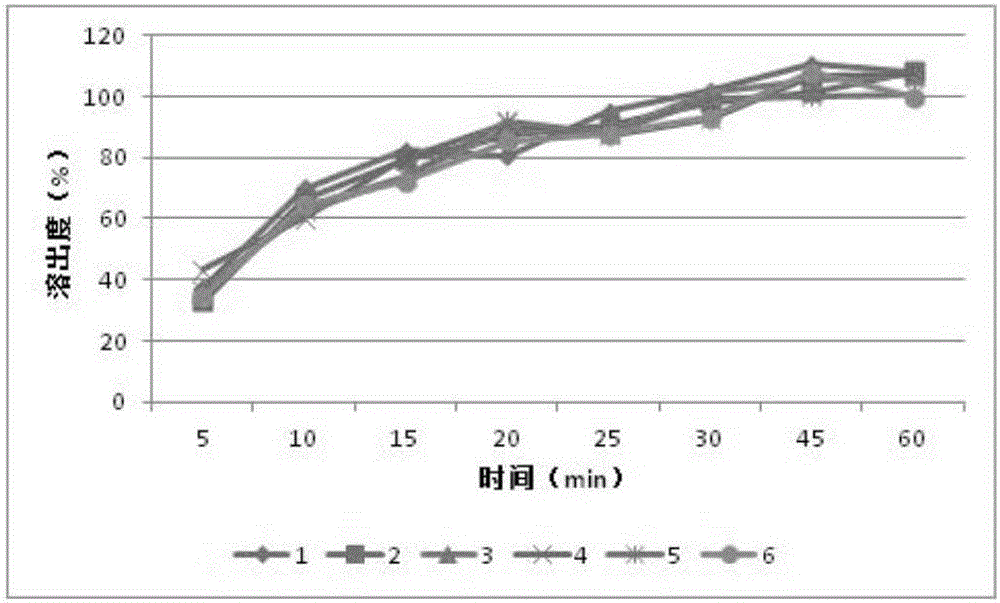
[0028] Example 2 Preparation of capsules containing orlistat and xylooligosaccharides
[0029] Prescription: (based on 1000 capsules):
[0030]
[0031]
[0032] Preparation Process:
[0033] Sodium lauryl sulfate, povidone K30, sodium carboxymethyl starch, microcrystalline cellulose, orlistat, and xylooligosaccharide powder were all passed through an 80-mesh sieve before use. Weigh the prescribed amount of sodium lauryl sulfate, povidone K30, sodium starch glycolate, microcrystalline cellulose, orlistat, and xylooligosaccharide powder, and first mix a small amount of sodium lauryl sulfate, polyvinyl Mix ketone K30 and carboxymethyl starch sodium evenly, then add microcrystalline cellulose, orlistat, and xylooligosaccharides, mix evenly, and pass through an 80-mesh sieve twice. Slowly add 50% ethanol solution containing 10% povidone K30 to the mixed powder to make a soft material, extrude and sieve through a 20-mesh granule, dry the wet granule in a blast drying oven a...
Embodiment 3
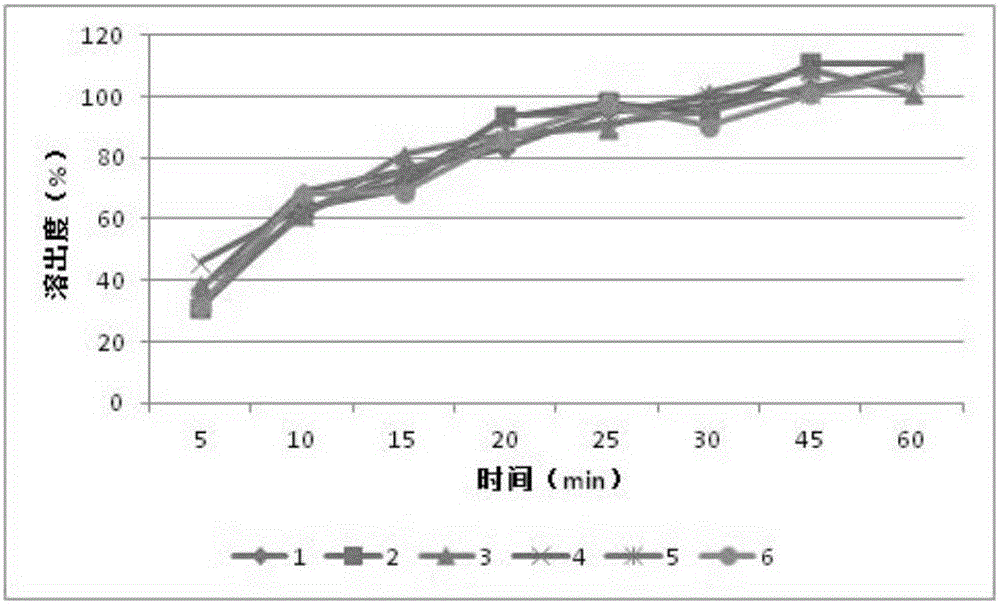
[0034] Example 3 Capsules containing orlistat and soybean oligosaccharides
[0035] Prescription: (based on 1000 capsules):
[0036]
[0037] Preparation Process:
[0038] Sodium lauryl sulfate, povidone K30, sodium starch glycolate, microcrystalline cellulose, orlistat, and soybean oligosaccharide powder are all passed through an 80-mesh sieve before use. Weigh the prescribed amount of sodium lauryl sulfate, povidone K30, sodium starch glycolate, microcrystalline cellulose, orlistat, soybean oligosaccharide powder, first mix a small amount of sodium lauryl sulfate, povidone Mix ketone K30 and carboxymethyl starch sodium evenly, then add microcrystalline cellulose, orlistat, and fructooligosaccharides, mix evenly, and pass through an 80-mesh sieve twice. Slowly add 50% ethanol solution containing 10% povidone K30 to the mixed powder to make a soft material, extrude and sieve through a 20-mesh granule, dry the wet granule in a blast drying oven at 30°C for 6 hours, and tak...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com