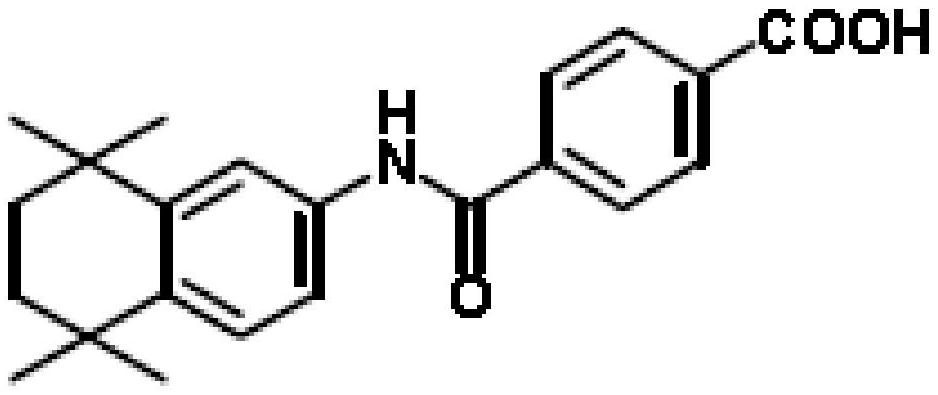
A kind of oral solid preparation of Tamibarotene, its preparation method and application
A technology of tamibarotene and solid preparation, applied in the field of medicine, can solve the problems of low dissolution rate, unqualified content uniformity of tablets or capsules, poor fluidity, etc., achieves good dissolution rate, improves bioavailability, and is suitable for The effect of clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] This example is used to illustrate the solid preparation of Tamibarotene of the present invention and its preparation method.
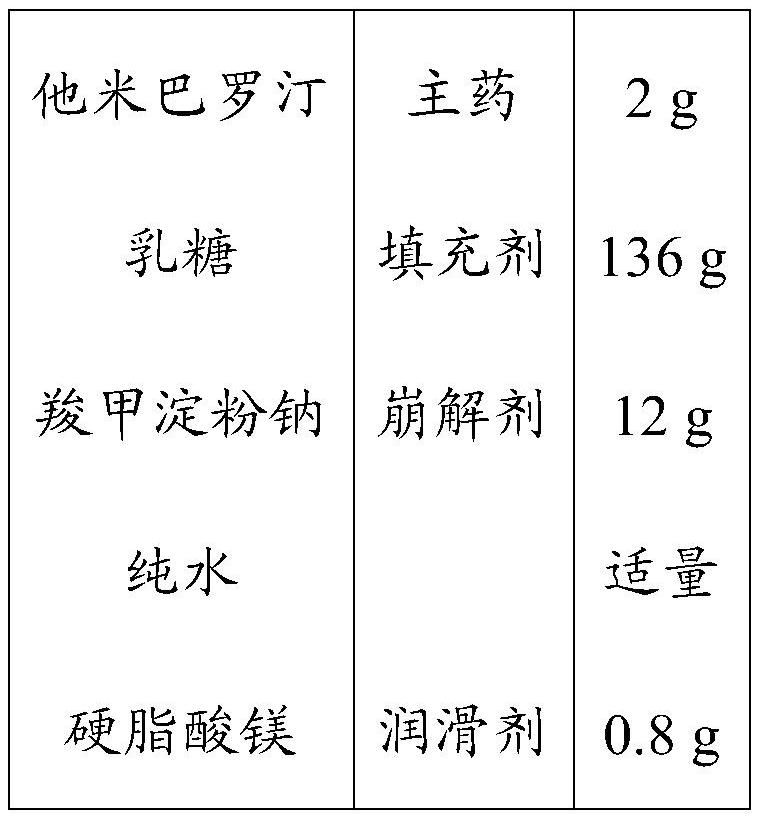
[0046] Tamibarotene solid preparations were prepared according to the following prescription.
[0047]
[0048]
[0049] Preparation:
[0050] (1) pulverizing the tamibarotene raw material, and sieving to obtain the tamibarotene;
[0051] (2) the filler, disintegrant and lubricant are sieved;
[0052] (3) After the filler and the disintegrant in the step (2) are mixed uniformly, they are uniformly mixed with the tamibarotene in the step (1) in equal increments;
[0053] (4) Add water to the mixture of the tamibarotene, filler and disintegrant in step (3) to make soft material, sieve and granulate;
[0054] (5) dry, whole grain is made into Tamibarotene granule;
[0055] (6) Add the lubricant to the tamibarotene granules in step (5) and mix evenly to prepare the solid preparation.
Embodiment 2
[0057] This example is used to illustrate the solid preparation of Tamibarotene of the present invention and its preparation method.
[0058] Tamibarotene solid preparations were prepared according to the following prescription.
[0059]
[0060]
[0061] Preparation method: prepare with reference to the method of Example 1.
Embodiment 3
[0063] This example is used to illustrate the solid preparation of Tamibarotene of the present invention and its preparation method.
[0064] Tamibarotene solid preparations were prepared according to the following prescription.
[0065]
[0066] Preparation method: prepare with reference to the method of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com