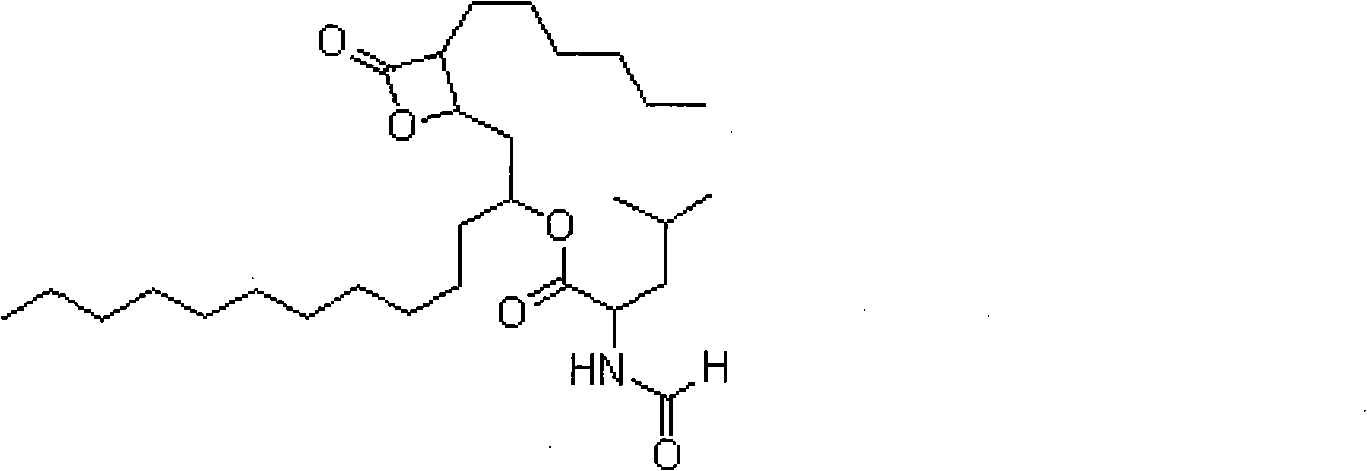
Method for preparing orlistat
A technology for orlistat and statin, applied in the field of extraction and purification of orlistat, can solve the problems of complex process steps, difficulty in obtaining high purity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add 100 liters of acetone to 100 liters of orlistat intermediate nipostatin fermentation broth (fermentation unit: 7 g / liter), stir and extract for 1 hour, and filter by plate and frame to obtain 190 liters of acetone extract. Filter through a titanium rod filter, adjust the pH to 3.0 with oxalic acid after filtration, and pack 20 liters of HZ820 macroporous adsorption resin into a column to absorb the acetone leaching solution after pretreatment, and the adsorption speed is 10 liters / hour. After the adsorption was completed, 60 liters of 80% acetone solution was used to elute, and the elution speed was 16 liters / hour, and the acetone eluate containing nipostatin was collected by TLC detection. After the elution was completed, 60 liters of acetone eluent containing nipostatin was obtained.
[0025] Add 20 liters of water to 60 liters of acetone eluent, adjust the acetone concentration to about 60%, add 20 liters of heptane for extraction, and after extraction for 1 hour...
Embodiment 2
[0030] After 500 liters of nipostatin fermentation broth (fermentation unit 7.2 g / liter) was stirred and leached with 750 liters of acetone for 1 hour, the plate and frame were filtered to obtain 1200 liters of acetone leaching solution, which was then filtered with a 0.1um titanium rod filter. After filtration, the pH was adjusted to 4.0 with oxalic acid, and 90 liters of HZ820 macroporous adsorption resin was packed into a column to absorb the acetone leaching solution after pretreatment, and the adsorption speed was 50 liters / hour. After adsorption, 350 liters of 80% acetone solution was used to elute, and the elution speed was 90 liters / hour, and the acetone eluate containing nipostatin was collected by TLC detection. After elution, 350 liters of acetone eluent containing nipostatin were obtained.
[0031] Add 118 liters of water to 340 liters of acetone eluent, adjust the acetone concentration to about 65%, add 120 liters of heptane for extraction for 1 hour, statically s...
Embodiment 3
[0036]Add 1,400 liters of acetone to 1,000 liters of nipostatin fermentation broth (fermentation unit 6.6 g / liter), and then add 1,400 liters of acetone to stir and extract for 1 hour, then filter to obtain 2,200 liters of acetone extract. Adjust the pH to 4.5, 160 liters of HZ818 macroporous adsorption resin is packed into a column, and after pretreatment, the acetone leaching solution is adsorbed at a speed of 80 liters / hour. liter / hour, and the acetone eluate containing nipostatin was collected by TLC detection. After the elution was completed, 640 liters of acetone eluent containing nipostatin were obtained.
[0037] Add 210 liters of water to 640 liters of acetone eluent, adjust the acetone concentration to about 60%, add 220 liters of heptane for extraction for 1 hour, statically separate layers, and concentrate the heptane extraction phase to obtain 8.95 kg of crude nipostatin, of which nipostatin The statin concentration was 58%.
[0038] 8.95 kg of crude nipostatin ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com