Orlistat purification process
A technology of orlistat and process, applied in the field of chemistry, can solve problems such as the inability to solve the purification requirements of key impurities of orlistat, the environmental pollution of organic solvents and solid waste, and the inability to guarantee product quality and production capacity requirements, etc., to achieve reduction Purchase price, good recovery, low column pressure effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Follow the steps below to purify orlistat
[0038] 1) Take 100 g of crude orlistat (content 89%), dissolve it in 70% methanol to a solution of 100 mg / mL, filter, and filter with a filter mesh of 100 mesh;
[0039] 2) Use DAC300 to prepare the column, match with 30μm C18 filler (C18 has a pore size of 80A, and a carbon load of 20%), and the total amount of the column is 6kg;
[0040] 3) Centrifuge at a centrifugal rate of 10000r / min, and take the supernatant;
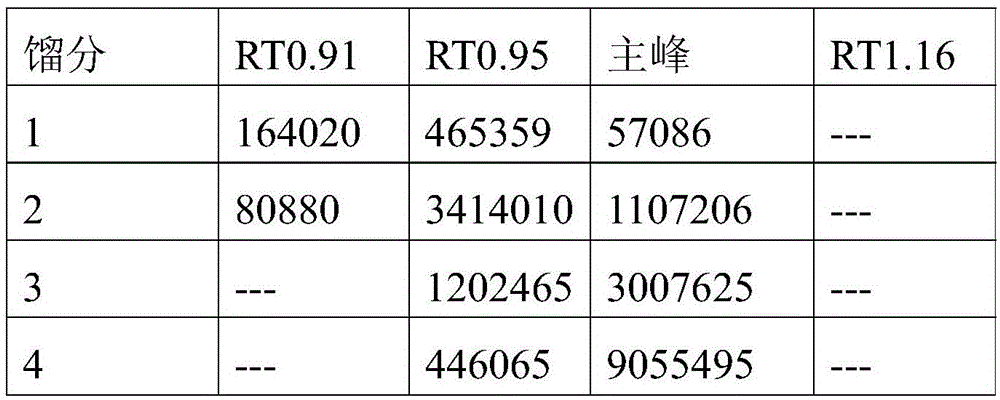
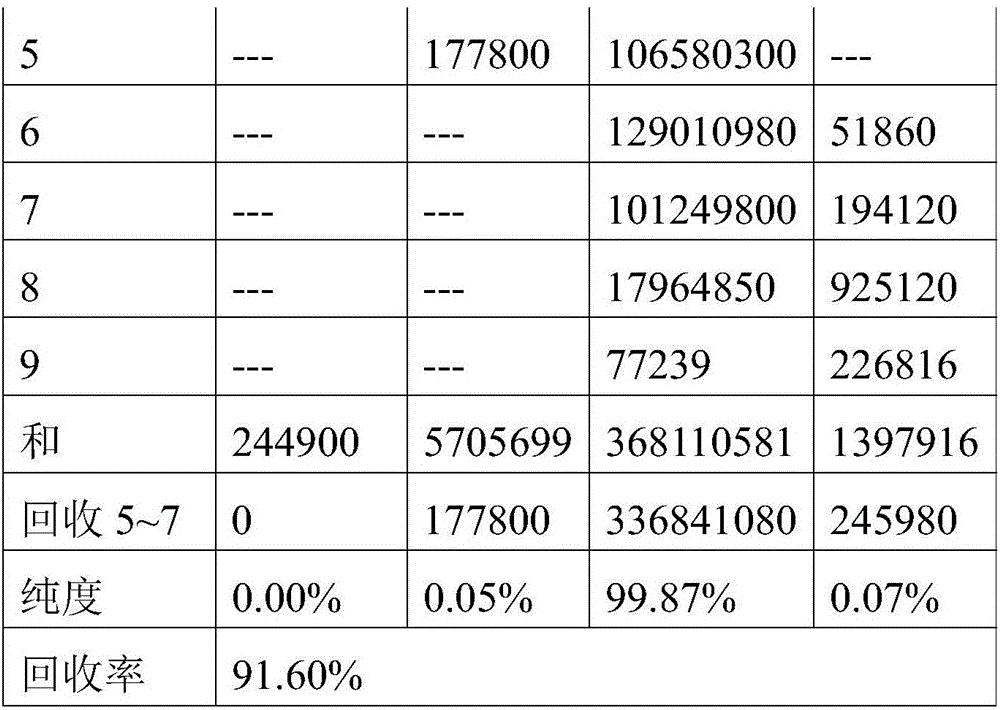
[0041]4) Inject the supernatant into the chromatographic system, and use an eluent to elute, wherein the eluent is a mixed solvent of ethanol and water 85:15, the equilibrium pressure is 2Mpa, the wavelength is 210nm, and the injection volume is 0.8%; For isocratic elution, the flow rate of the eluent is 1550mL / min, and the elution time is 50min;
[0042] 5) Collect the eluate from the target section, concentrate it in vacuum at 50°C, the vacuum degree is -0.04Mpa, and then dry it in vacuum at 60°C until the moi...
Embodiment 2
[0044] Follow the steps below to purify orlistat
[0045] 1) Take 500 g of crude orlistat (content 90%), dissolve it in 90% methanol to a solution of 10 mg / mL, filter, and filter with a filter mesh of 100 mesh;
[0046] 2) Use DAC300 to prepare the column, match with 30μm C18 filler (C18 has a pore size of 120A, and a carbon load of 12%), and the total amount of the column is 6kg;
[0047] 3) Centrifuge at a centrifugal rate of 15000r / min, and take the supernatant;
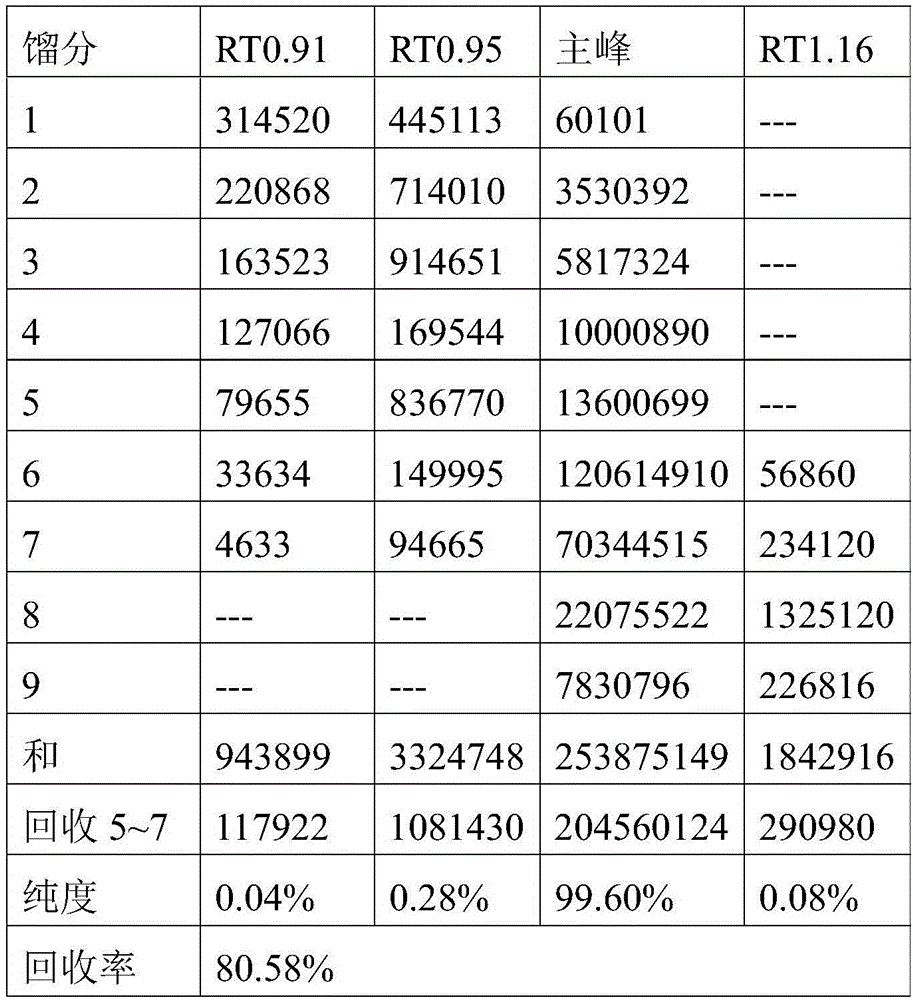
[0048] 4) inject the supernatant into the chromatographic system, and use the eluent to elute, wherein the eluent is a mixed solvent of isopropanol and water 80:20, the equilibrium pressure is 5Mpa, the wavelength is 210nm, and the injection volume is 1.5%; The removal is isocratic elution, the flow rate of the eluent is 600mL / min, and the elution time is 60min;
[0049] 5) Collect the eluate from the target section, concentrate it in vacuum at 50°C, the vacuum degree is -0.04Mpa, and then dry it in vacuum at 70...
Embodiment 3
[0051] Follow the steps below to purify orlistat
[0052] 1) Take 300 g of crude orlistat (content 91%), dissolve it in 70% methanol to a solution of 100 mg / mL, filter, and filter with a mesh number of 100;
[0053] 2) Use DAC300 to prepare the column, match with 25μm C18 filler (the pore size of C18 is 200A, and the carbon load is 8%), and the total volume of the column is 6kg;
[0054] 3) Centrifuge at a centrifugal rate of 15000r / min, and take the supernatant;
[0055] 4) Inject the supernatant into the chromatographic system, and use an eluent to elute, wherein the eluent is a mixed solvent of methanol and water 88:12, the equilibrium pressure is 2Mpa, the wavelength is 210nm, and the injection volume is 1.5%; For isocratic elution, the flow rate of eluent is 600mL / min, and the elution time is 60min;
[0056] 5) Collect the eluate from the target section, concentrate it in vacuum at 50°C, the vacuum degree is -0.04Mpa, and then dry it in vacuum at 60°C until the moisture...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com