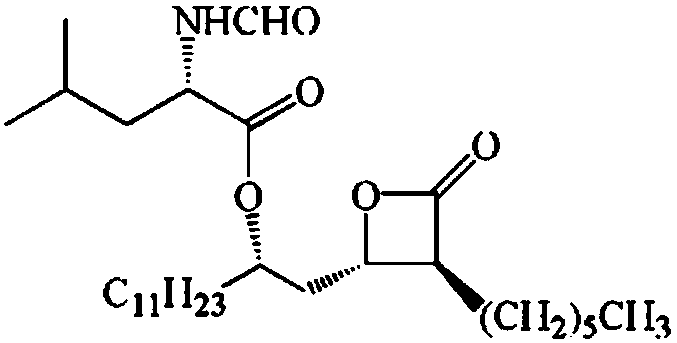
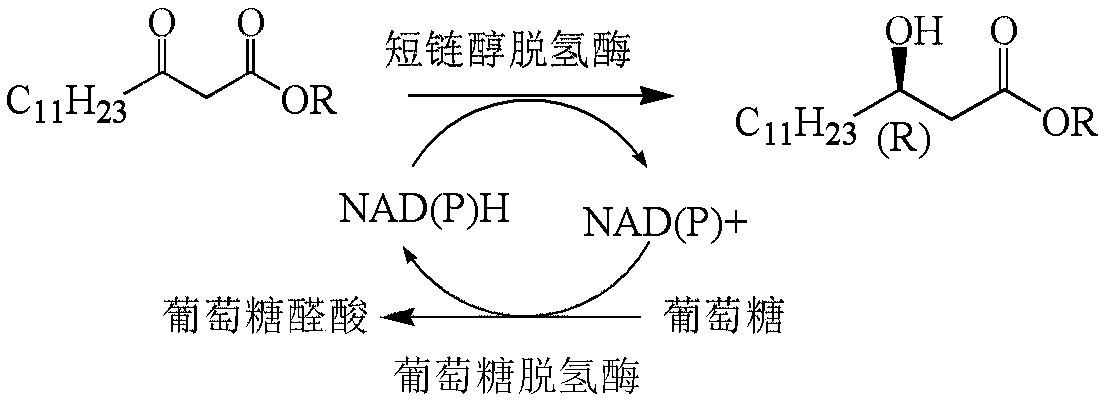
Method for preparing orlistat intermediate by enzymatic technology
A technology for orlistat and enzymatic preparation, which is applied in the field of enzymatic preparation of orlistat intermediates, can solve the problems of high production equipment requirements, high catalyst cost, high corrosion, etc., and achieve an environmentally friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 5 g of the substrate β-carbonyltetradecanoic acid methyl ester was added to a 250 mL reactor, and subsequently, 100 mL containing 0.1 g / L NAD + , 30g / L cell disruption solution containing recombinant short-chain alcohol dehydrogenase, 10g / L cell disruption solution containing recombinant glucose dehydrogenase, 100g / L glucose, and 0.1M phosphate buffer (pH 7.0) were added to the reactor In the process, the solid-liquid ratio of β-carbonyltetradecanoic acid methyl ester to the mixed solution is 50g / L, and the pH automatic control system and 1M Na 2 CO 3 The solution controls the pH of the enzyme reaction to around 7.0. React at 35°C for 12h. The substrate conversion rate is greater than 98%, the product concentration is 49.0g / L, and the chiral ee value of the product is greater than 99%.
Embodiment 2
[0043] 7.5 g of the substrate β-carbonyltetradecanoic acid methyl ester was added to a 250 mL reactor, and subsequently, 100 mL containing 0.1 g / L NAD + , 35g / L cell disruption solution containing recombinant short-chain alcohol dehydrogenase, 10g / L cell disruption solution containing recombinant glucose dehydrogenase, 100g / L glucose, and 0.1M phosphate buffer (pH 7.0) were added to the reactor Among them, the solid-liquid ratio of β-carbonyltetradecanoic acid methyl ester to the mixed solution is 75g / L, and the pH automatic control system and 1M Na 2 CO 3 The solution controls the pH of the enzyme reaction to around 7.0. React at 35°C for 12h. The substrate conversion rate is greater than 96%, the product concentration is 72.0g / L, and the chiral ee value of the product is greater than 99%.
Embodiment 3
[0045] 10 g of the substrate β-carbonyltetradecanoic acid methyl ester was added to a 250 mL reactor, and subsequently, 100 mL containing 0.1 g / L NAD + , 30g / L cell disruption solution containing recombinant short-chain alcohol dehydrogenase, 10g / L cell disruption solution containing recombinant glucose dehydrogenase, 200g / L glucose, and 0.1M phosphate buffer (pH 7.0) were added to the reactor In the process, the solid-liquid ratio of β-carbonyltetradecanoic acid methyl ester to the mixed solution is 100g / L, and the pH automatic control system and 1M Na 2 CO 3 The solution controls the pH of the enzyme reaction to around 7.0. React at 35°C for 12h. The substrate conversion rate is greater than 78%, the product concentration is 78.0g / L, and the chiral ee value of the product is greater than 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com