A kind of preparation method of crizotinib chiral intermediate
A technology of crizotinib and intermediates, applied in the field of non-small cell lung cancer drugs, can solve the problems of difficult industrialization, complicated operation, expensive reagent yield, etc., and achieve solvent recyclability, simple operation, and stable process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: the preparation of compound (2)
[0034] Add 500 g (2.42 mol) of 1-(2,6-dichloro-3-fluorophenyl) ethyl ketone into 1500 ml of methanol, add 101 g (2.65 mol) of sodium borohydride under stirring in an ice bath, and control the temperature at 0-5 ℃, the addition was completed, stirred at room temperature for 3 hours, concentrated methanol under reduced pressure, added water and dilute hydrochloric acid to adjust the pH to 6, extracted with ethyl acetate, dried the organic phase, and concentrated under reduced pressure to obtain 500 g of a colorless liquid. Add 500 grams of this colorless liquid into 2500 ml of dichloromethane, add 351 ml (2.53 mol) of triethylamine and 196 ml (2.53 mol) of methanesulfonyl chloride under ice-bath cooling, stir at room temperature for 3 hours, add water to extract, and depressurize the organic phase after drying Concentration gave 650 g of light yellow liquid. 650 grams of this yellow liquid was dissolved in 3500ml DMF, added...
Embodiment 2
[0036] Embodiment 2: the preparation of compound (1) resolution salt
[0037]At 70°C, dissolve 20 grams (66 mmol) of 3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)pyridin-2-amine in 200ml of 80% ethanol, add L-diphenylmethane 23.8 grams (66mmol) of acyl tartaric acid, heated to reflux and stirred for 2 hours and then slowly lowered to room temperature, stirred for 2 hours, filtered, added 200ml of 80% ethanol to the filter cake, heated and refluxed and stirred for two hours and allowed to stand down to room temperature for 2 hours. After filtration, 17.3 g (26.2 mmol) of L-dibenzoyl tartrate salt of compound (1) was obtained as a solid. Yield: 40%. Melting point: 160.7-168.7℃(dec),[α] 20 D =-138.3° (c=1, DMSO) 1 HNMR (300MHz, DMSO) δ: 8.02(d, J=9.0Hz, 4H), 7.73(t, J=6.0Hz, 2H), 7.58(m, 5H), 7.45(m, 2H), 6.63(d, J=6.0Hz, 1H), 6.42(dd, J=6.0Hz, 1H), 6.15(br, 2H), 5.97(q, J=2.0Hz, 1H), 5.85(s, 2H), 1.75(s, 3H)
Embodiment 3
[0038] Embodiment 3: the preparation of compound (1)
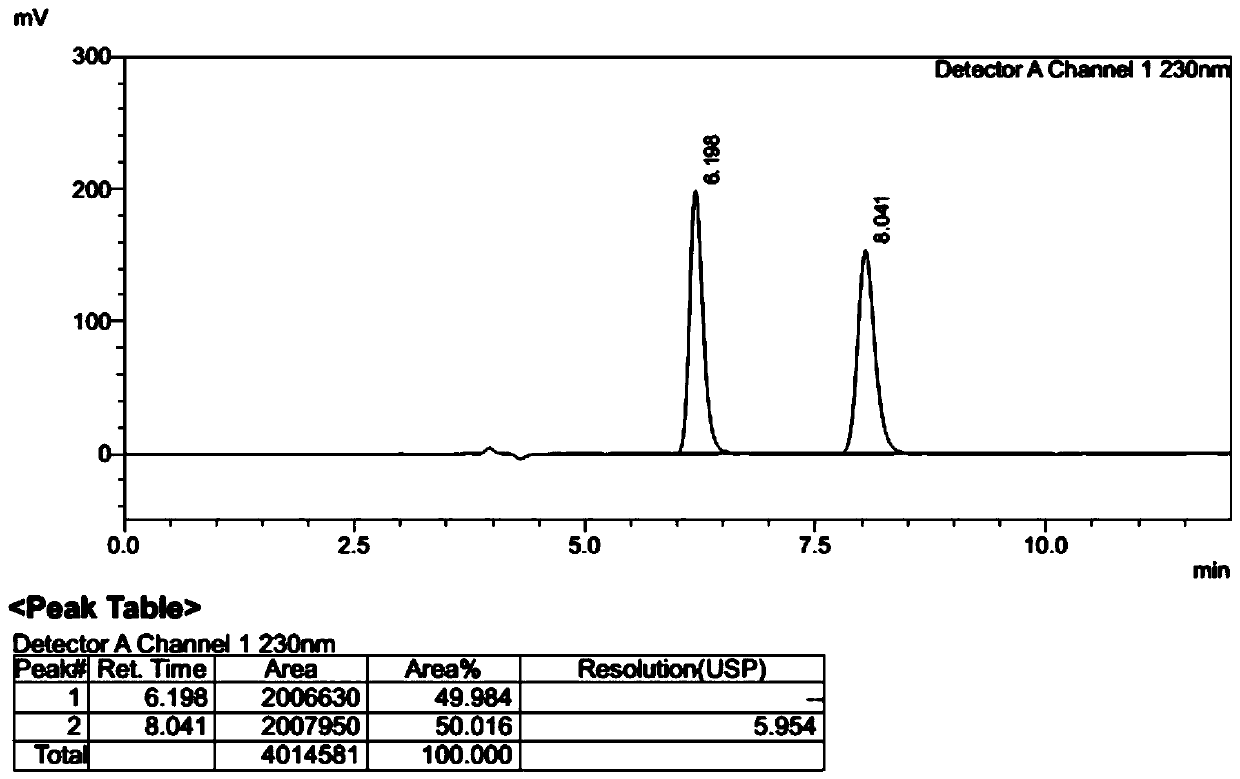
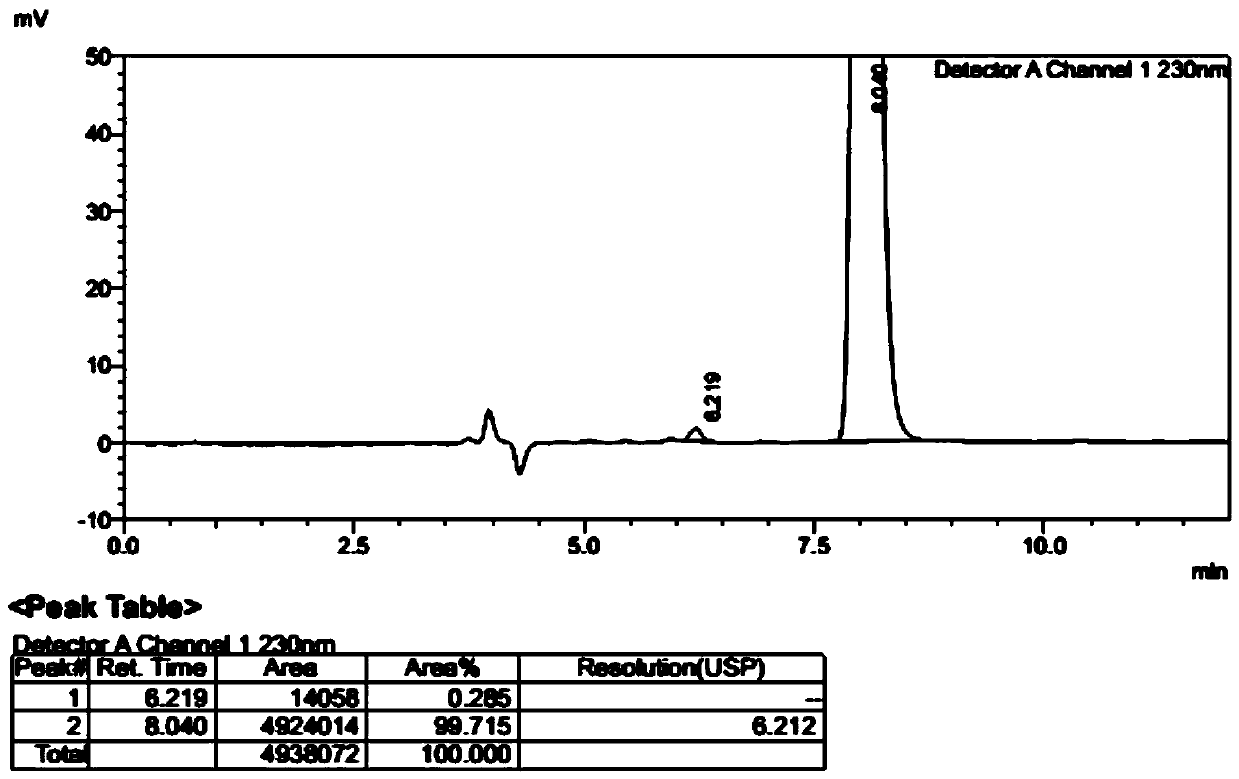
[0039] With 17.3 grams (26.2 mmol) of L-dibenzoyl tartrate of compound (1) obtained in Example 2, add ethyl acetate 100 ml, water 100 ml, add dropwise 10% sodium hydroxide solution at room temperature, adjust pH =10, after the layers were separated, the organic phase was washed once with 50 ml of saturated brine, and the layers were separated. Ethyl acetate was dried over anhydrous sodium sulfate for 2 hours, filtered, and the filtrate was concentrated under reduced pressure at 40°C to obtain 7.5 g of white solid compound (1), yield: 95%. Sampling and detection showed that the HPLC purity was 99.9%, and the optical purity ee: 99.4%. 1 HNMR (300MHz, CDCl 3 )δ: 7.60(d, J=4.8Hz, 1H), 7.29(m, 1H), 7.05(t, J=8.4Hz, 1H), 6.70(d, J=7.7Hz, 1H), 6.46(dd, J=5.3,2.28Hz,1H),6.01(q,J=6.6Hz,1H),5.2(brs,2H),1.82(d,J=6.7Hz,3H).Mp:118.1-119.2℃[α] 20 D =-184.2° (c=2, CHCl 3 ). [Chiral HPLC method: chromatographic column CHIRALCEL OD ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com