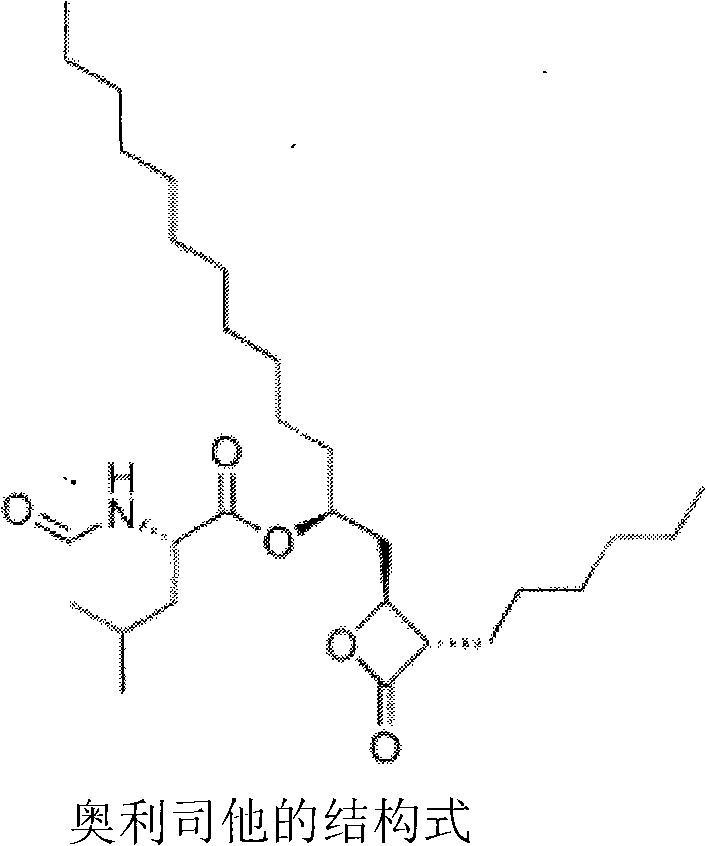
Method for separating and purifying Orlistat
A technology of orlistat and mobile phase, which is applied in the field of separation and purification, can solve the problems of high sample loss rate, large environmental pollution, and small purification range, and achieve the effects of less solvent consumption, less environmental pollution in operation, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Dissolving and filtering:
[0040] According to the ratio of orlistat crude product solid mass:solvent volume=10g:1L, orlistat crude product solid is dissolved with mobile phase, after dissolving, filter with organic microporous membrane of 0.45 μm, the filtrate is prepared as the next step to purify orlistat Raw material of lixistat.
[0041] The purity of the orlistat of the crude product used is 96% (HPLC area normalization method), and the maximum simple impurity is 0.6% (HPLC does not add the principal component self-contrast method of correction factor, Chinese Pharmacopoeia 2010 edition, P appendix 31), flow Phase select methanol-water solution with a volume ratio of 86:14
[0042] (2) separation and purification:
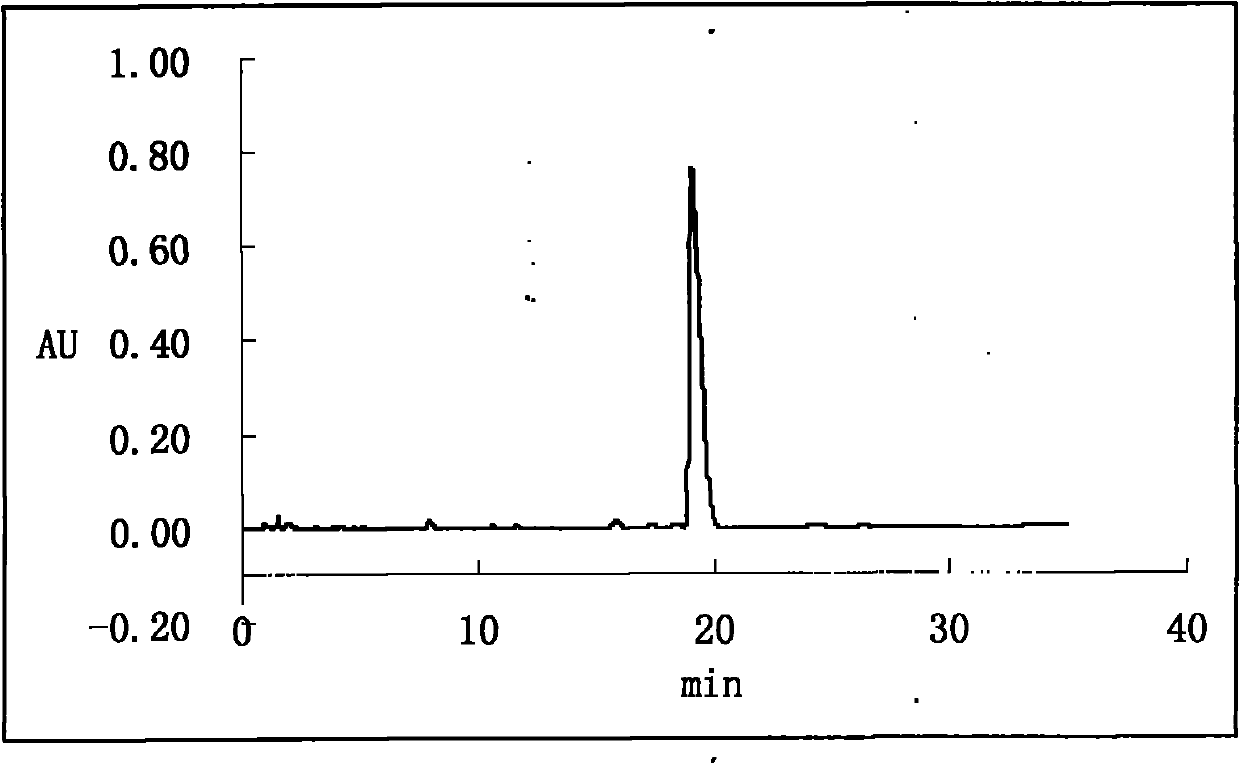
[0043] Inject 2L of the filtrate in step (1) into a reversed-phase high-performance preparative liquid chromatograph, elute isocratically, and detect online with an ultraviolet detector, and collect orlistat preparation components in a targeted ...
Embodiment 2
[0048] (1) Dissolving and filtering:
[0049] According to the ratio of orlistat crude product solid mass:solvent volume=15g:1L, orlistat crude product solid is dissolved with mobile phase, after dissolving, filter with 0.45 μ m organic microporous membrane, and the filtrate is used as the next step to prepare purified orlistat Raw material of lixistat.
[0050] The purity of the orlistat of the crude product used is 97% (HPLC area normalization method), and the maximum simple impurity is 0.45% (HPLC does not add the principal component self-contrast method of correction factor, Chinese Pharmacopoeia 2010 edition, P appendix 31),
[0051] Acetonitrile-water solution with a volume ratio of 80:20 was selected as the mobile phase.
[0052] (2) separation and purification:
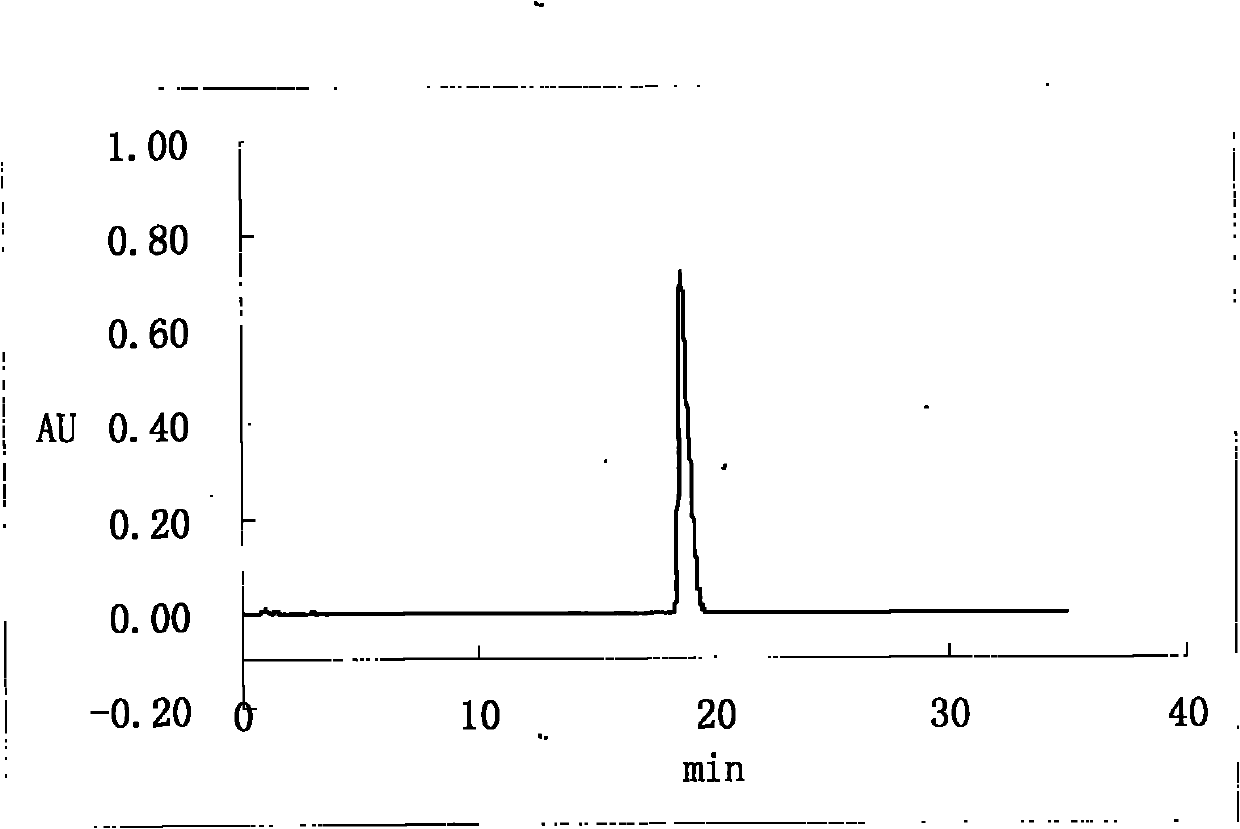
[0053] Inject 2.5 L of the filtrate in step (1) into a reversed-phase high-efficiency preparative liquid chromatograph, elute isocratically, and detect online with an ultraviolet detector to collect orlistat...
Embodiment 3
[0058] (1) Dissolving and filtering:
[0059] According to the ratio of orlistat crude product solid mass:solvent volume=20g:1L, orlistat crude product solid is dissolved with mobile phase, after dissolving, filter with 0.45 μ m organic microporous membrane, and the filtrate is used as the next step to prepare purified orlistat Raw material of lixistat.
[0060] The purity of the orlistat of the crude product used is 98% (HPLC area normalization method), and the maximum simple impurity is 0.44% (HPLC does not add the principal component self-contrast method of correction factor, Chinese Pharmacopoeia 2010 edition, P appendix 31),
[0061] The mobile phase was ethanol-water solution with a volume ratio of 88:12.
[0062] (2) separation and purification:
[0063] Inject 2.5 L of the filtrate in step (1) into a reversed-phase high-efficiency preparative liquid chromatograph, elute isocratically, and detect online with an ultraviolet detector to collect orlistat preparation comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com