Argatroban injection and preparation method thereof
A technology of argatroban and injection, which is applied in the field of pharmaceutical preparations, can solve the problems of not being suitable for large-scale production in workshops and complicated preparation methods, and achieve the effects of increased drug use risk, good stability, and low drug risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
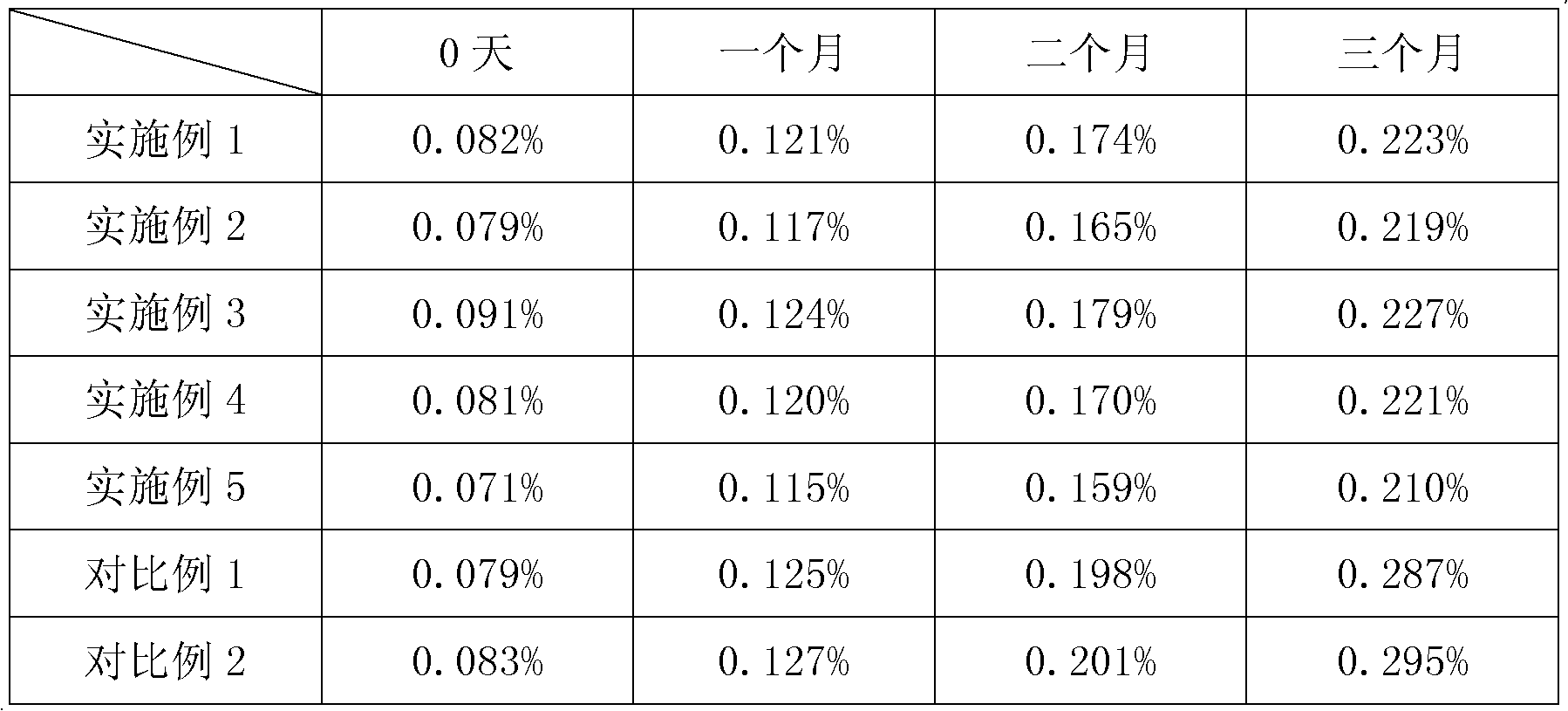
Embodiment 1
[0027] The preparation of embodiment 1 argatroban injection
[0028] Weigh 80g of D-sorbitol, add 5000mL of water for injection, stir to dissolve, add 0.1% activated carbon for needles (5.0g) according to the mass volume ratio, stir for 15 minutes, filter the liquid through a 1um titanium rod to decarbonize.
[0029] Add 1 part of argatroban to the above filtrate, stir to suspend the argatroban in the liquid medicine, heat the liquid medicine to 60°C, and stir until the argatroban is completely dissolved. Add water for injection to the above medicinal solution to 10000 parts, circulate and filter to mix evenly, and adjust the pH of the medicinal solution to 4.2 with 0.1mol / L hydrochloric acid solution. The medicinal solution is sterilized and filtered through a 0.22um microporous membrane, filled in brown glass ampoules, and filled with nitrogen gas before filling. Melt seal, check for leaks, and steam sterilize at 121°C for 20 minutes.
Embodiment 2
[0030] The preparation of embodiment 2 argatroban injection
[0031] Weigh 100g of D-sorbitol, add 1000mL of water for injection, stir to dissolve, add 0.1% activated carbon for needles (1.0g) according to the mass volume ratio, stir for 15 minutes, filter the liquid through a 1um titanium rod for decarbonization.
[0032] Add 1 part of argatroban to the above filtrate, stir to suspend the argatroban in the liquid medicine, heat the liquid medicine to 80°C, and stir until the argatroban is completely dissolved. Add water for injection to the above medicinal solution to 2000 parts, circulate and filter to mix evenly, and adjust the pH of the medicinal solution to 5.5 with 1mol / L sodium bicarbonate solution. The medicinal solution is sterilized and filtered through a 0.22um microporous membrane, filled in brown glass ampoules, and filled with nitrogen gas before filling. Melt seal, check for leaks, and steam sterilize at 121°C for 20 minutes.
Embodiment 3
[0033] The preparation of embodiment 3 argatroban injection
[0034] Weigh 150g of D-sorbitol, add 500mL of water for injection, stir to dissolve, add 0.1% activated carbon for needles (0.5g) according to the mass volume ratio, stir for 15 minutes, filter the liquid through a 1um titanium rod for decarbonization.
[0035] Add 1 part of argatroban to the above filtrate, stir to suspend the argatroban in the liquid medicine, heat the liquid medicine to 80°C, and stir until the argatroban is completely dissolved. Add water for injection to the above medicinal solution to 1000 parts, circulate and filter to mix evenly, and adjust the pH of the medicinal solution to 6.0 with 1mol / L sodium carbonate solution. The medicinal solution is sterilized and filtered through a 0.22um microporous membrane, filled in brown glass ampoules, and filled with nitrogen gas before filling. Melt seal, check for leaks, and steam sterilize at 121°C for 20 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com