Linezolid injection and preparation method thereof
A technology for linezolid and injection, applied in the field of linezolid injection and its preparation, capable of solving the problems of linezolid injection product quality and medication safety, and not proposing to reduce linezolid injection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
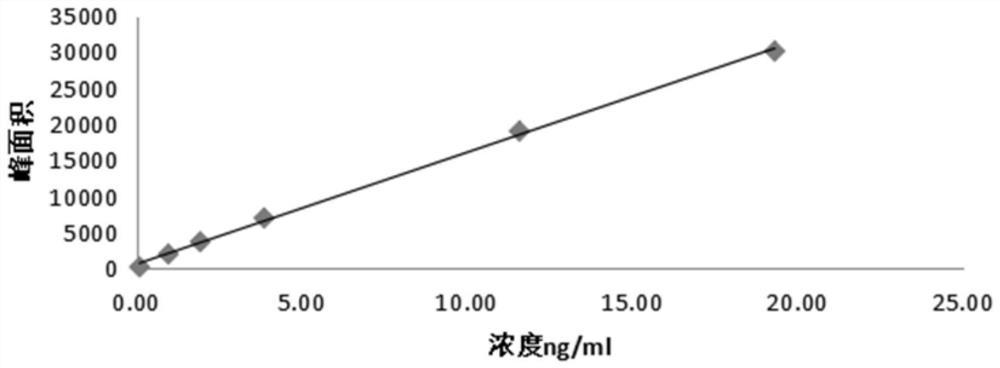
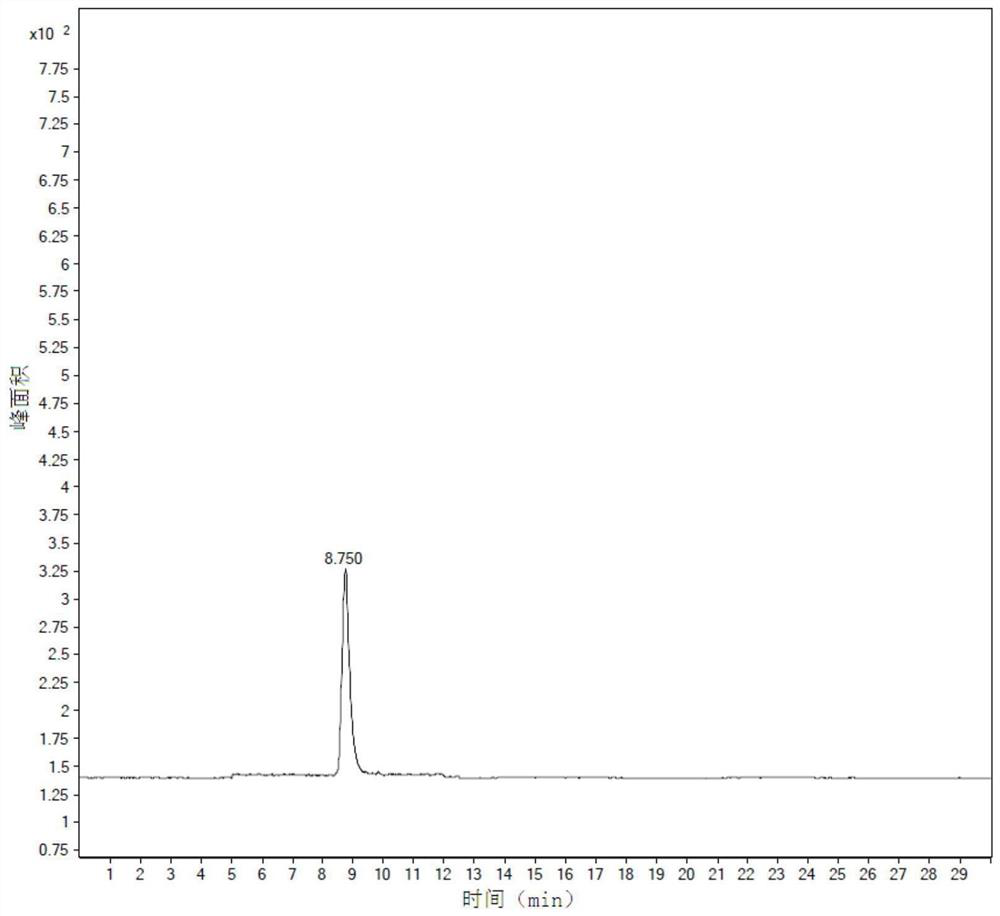
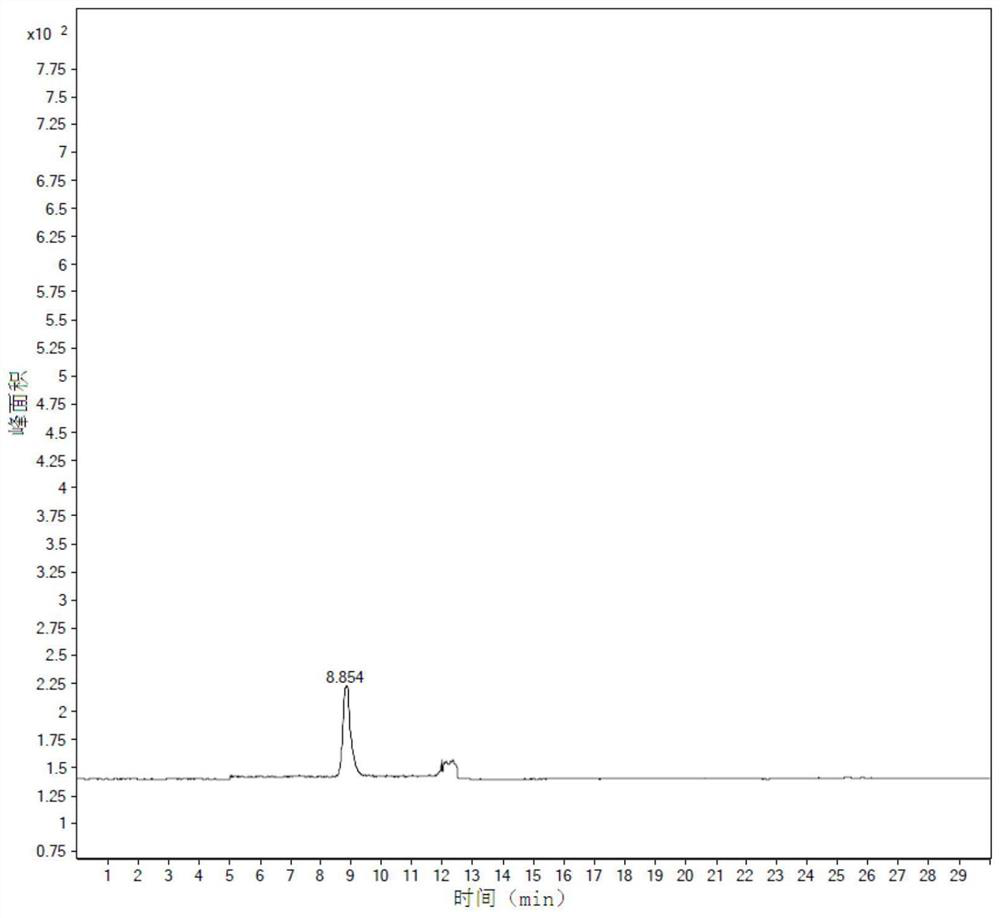
Image
Examples
Embodiment 1
[0043] A linezolid injection, the prescription is as follows:
[0044]
[0045]
[0046] The preparation method of the linezolid injection, the steps are as follows:
[0047] (1) Washing rubber plugs: Use a fully automatic rubber plug cleaning machine to clean the rubber plugs;
[0048] (2) Weighing: Weigh the prescribed amount of linezolid, anhydrous citric acid, sodium hydroxide and sodium chloride respectively;
[0049] (3) Preparation: Add water for injection to 90% of the full amount in the concentrated preparation tank, control the temperature at 60-65°C, and fill nitrogen under the liquid surface (that is, the nitrogen input port is located under the liquid surface to fill the liquid with nitrogen) , the nitrogen pressure is 0.3MPa, and when the dissolved oxygen in the water for injection is less than 2ppm, put the weighed anhydrous citric acid and sodium hydroxide into the concentrated tank, stir to dissolve completely, and add the weighed line Stir to dissolve...
Embodiment 2
[0057] A kind of linezolid injection, prescription is the same as embodiment 1, the preparation method of this linezolid injection, the steps are as follows:
[0058] (1) Washing rubber plugs: Use a fully automatic rubber plug cleaning machine to clean the rubber plugs;
[0059] (2) Weighing: Weigh the prescribed amount of linezolid, anhydrous citric acid, sodium hydroxide and sodium chloride respectively;
[0060] (3) Preparation: Add water for injection to 90% of the total amount in the concentrated preparation tank, control the temperature at 60-65°C, fill the liquid with nitrogen, the nitrogen pressure is 0.3MPa, and detect when the dissolved oxygen in the water for injection is <2ppm Put the weighed anhydrous citric acid and sodium hydroxide into the concentrated mixing tank, stir to dissolve completely, add the weighed linezolid, stir to dissolve completely, then add the weighed sodium chloride , stir to make it completely dissolve, keep the temperature of the liquid me...
Embodiment 3
[0068] A kind of linezolid injection, prescription is the same as embodiment 1, the preparation method of this linezolid injection, the steps are as follows:
[0069] (1) Washing rubber plugs: Use a fully automatic rubber plug cleaning machine to clean the rubber plugs;
[0070] (2) Weighing: Weigh the prescribed amount of linezolid, anhydrous citric acid, sodium hydroxide and sodium chloride respectively;
[0071] (3) Preparation: Add water for injection to 90% of the total amount in the concentrated preparation tank, control the temperature at 60-65°C, put weighed anhydrous citric acid and sodium hydroxide into the concentrated preparation tank, stir to make them completely Dissolve, add the weighed linezolid, stir to dissolve completely, then add the weighed sodium chloride, stir to dissolve completely, keep the liquid temperature at 60-65°C during the preparation process, transfer the liquid To dilute the tank, make up the water for injection to the full amount, stir unti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com