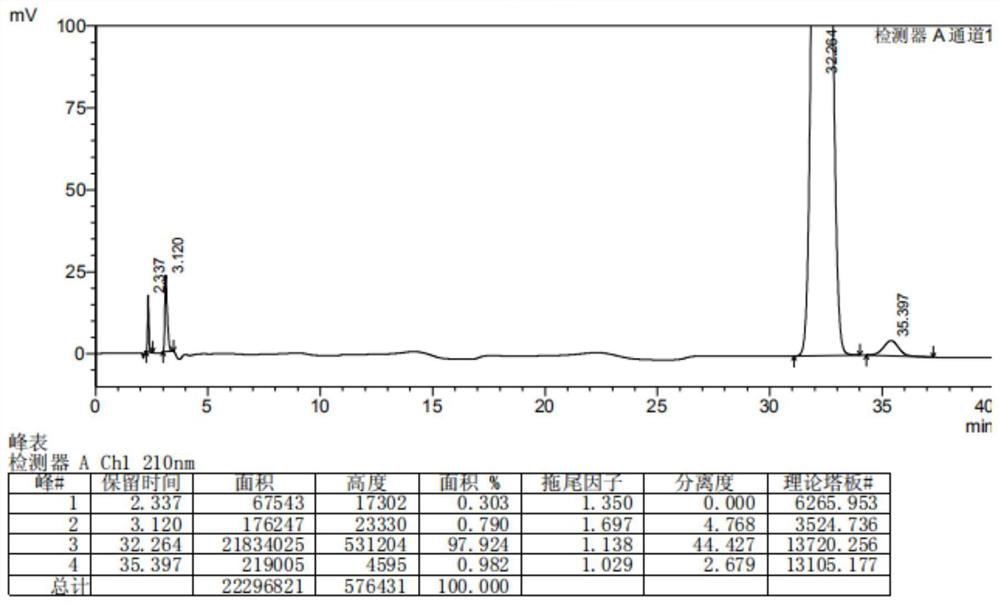
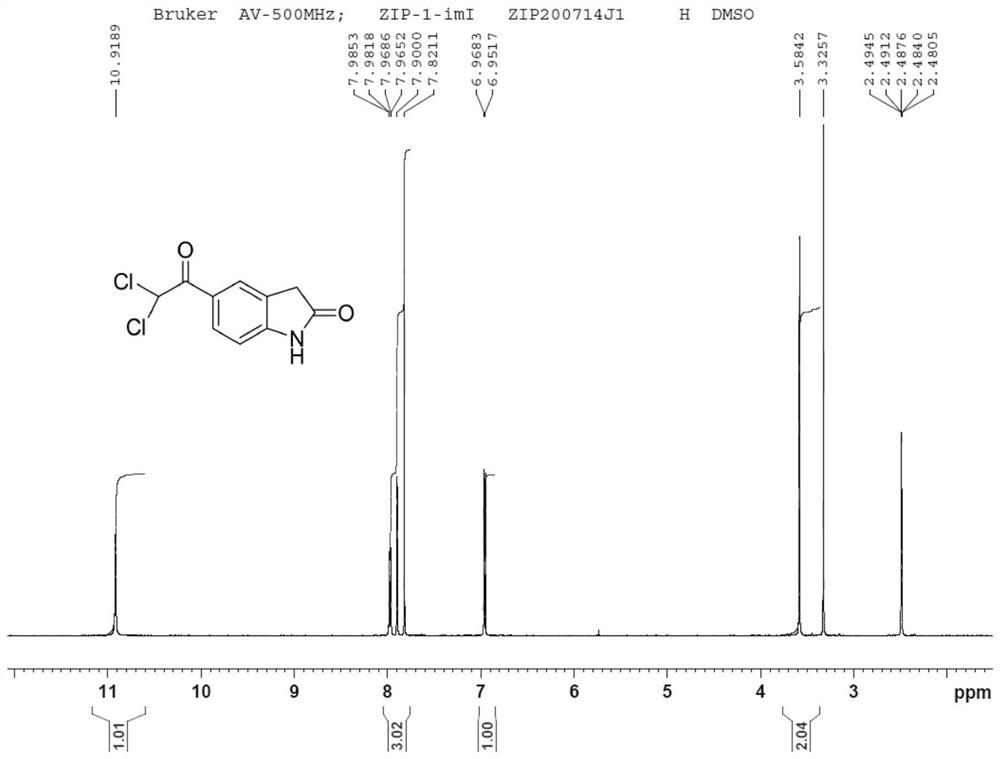
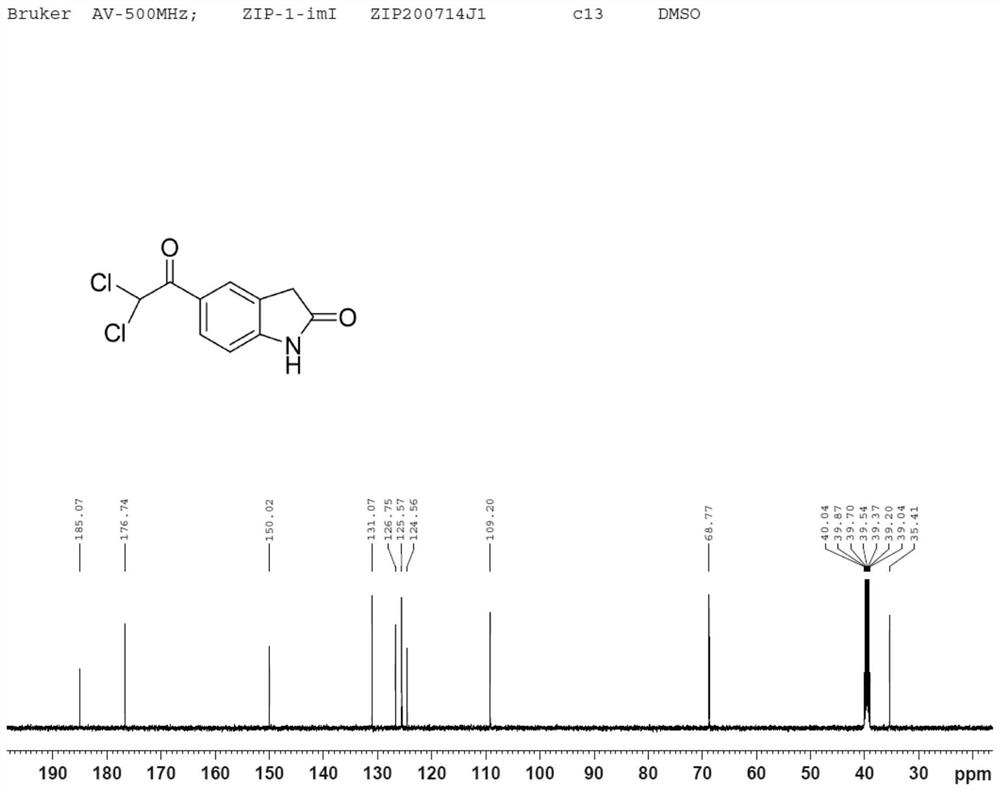
Dihalide impurity in ziprasidone hydrochloride intermediate and preparation method of dihalide impurity
A technology for ziprasidone hydrochloride and intermediates, which is applied in the field of ziprasidone hydrochloride impurity research, can solve problems such as carry-in and generation of impurities, and achieve the effects of strong controllability, mild conditions, and reduced drug risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0086] In the present invention, the ziprasidone is preferably prepared by the following steps:
[0087] Using 6-chloroindol-2-one as the starting material, introduce chloroacetyl chloride or bromoacetyl bromide through Friedel-Crafts reaction to obtain 5-(2-chloroacetyl)-6-chloro-1,3-di Hydrogen-indol-2-one or 5-(2-bromoacetyl)-6-chloro-1,3-dihydro-indol-2-one, followed by reduction of the carbonyl to give 5-(2-chloroethyl )-6-chloro-1,3-dihydro-indol-2-one or 5-(2-bromoethyl)-6-chloro-1,3-dihydro-indol-2-one, then in In the presence of a base, react with 3-piperazinyl-1,2-benzisothiazole hydrochloride to obtain ziprasidone.
[0088] The present invention provides a kind of preparation method of the dihalogen impurity in the ziprasidone hydrochloride intermediate, preferably comprises the following steps:
[0089] 1) After mixing the base and the first solvent, under a protective atmosphere, add dimethyl malonate, and then add 1-halo-2-nitrobenzene for nucleophilic substitu...
Embodiment 1
[0138] step 1:
[0139] Tetrahydrofuran (100mL, 10V) was put into a 250mL three-neck reaction flask, cooled to 0°C in an ice-water bath, sodium hydride (4.7g, 118.4mmol, 60%) was added in batches, and the temperature was controlled below 10°C. Under the protection of nitrogen, dimethyl malonate (10.4 g, 70.9 mmol) was started to be added dropwise, and the temperature was controlled below 15°C for 20 minutes. After the dropwise addition was completed, the mixture was stirred at 0-5°C for half an hour, and then compound 2 (10.0 g, 70.9 mmol) was added. The ice-water bath was removed, the temperature was raised to reflux (68° C.), and the mixture was stirred at reflux for 16 hours. TLC detection shows that the raw material still has a small amount of residue. Pour the reaction solution into 200g of crushed ice, add 300mL of dichloromethane, stir, dissolve and separate for extraction, then extract the aqueous phase once with 300mL of dichloromethane, combine the organic phases...
Embodiment 2
[0156] The difference from Example 1 is that the equivalent ratio of compound 4 and aluminum trichloride in step 4 is 1.0:2.5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com