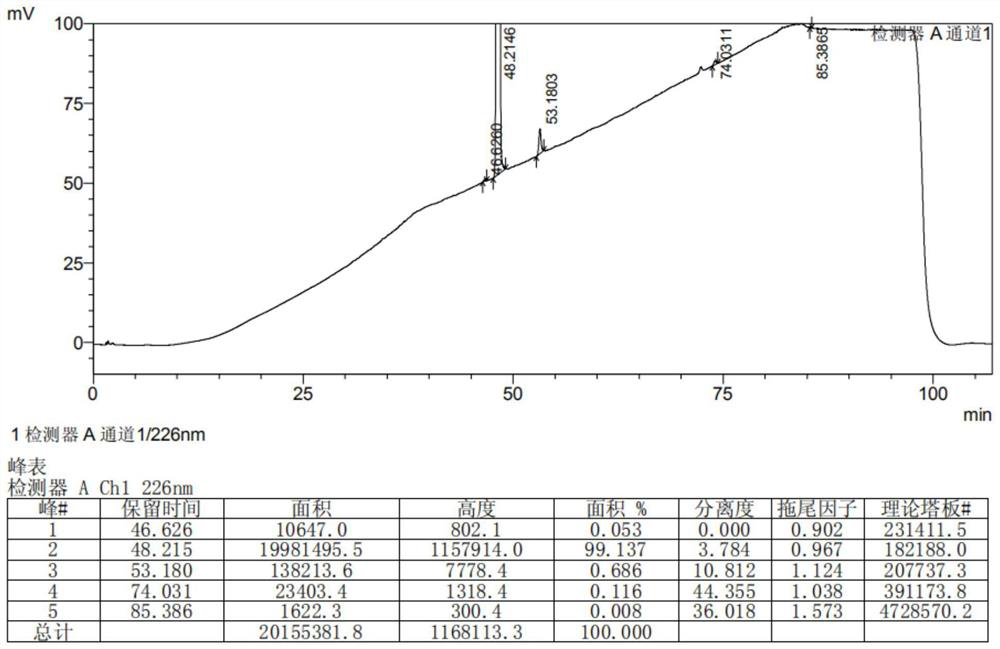
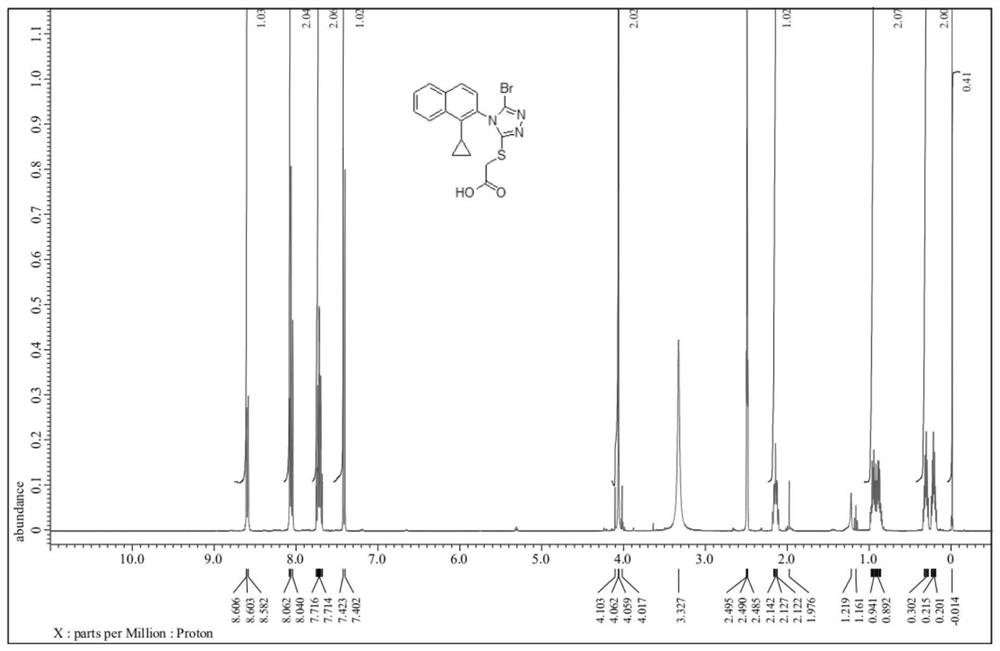
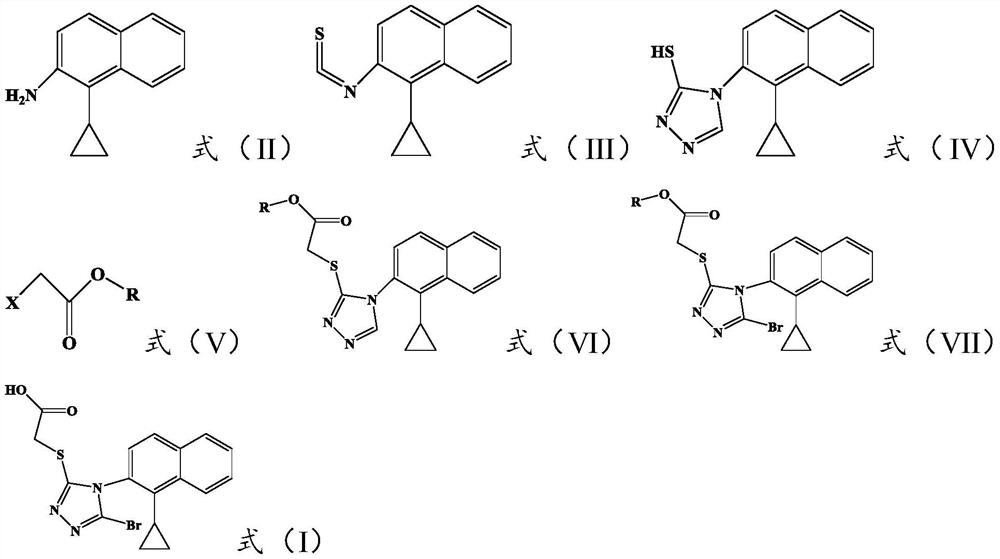
Preparation method of lesinurad related impurities
A technology for impurities and acidic substances, which is applied in the field of preparation of impurities related to Recinade, can solve the problems of difficult separation by column chromatography and difficult removal of impurities.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The invention provides a method for preparing Lesinald-related impurities, comprising the following steps: S1) nitrating 1-cyclopropylnaphthalene to obtain a nitrated mixture; S2) reducing the nitrated mixture to obtain reducing the mixture; S3) mixing the reducing mixture with the acidic substance in the first organic solvent, and performing the first cooling and crystallization to obtain the compound represented by the formula (II); S4) combining the compound represented by the formula (II) The compound shown in formula (III) is reacted with thiophosgene to obtain the compound shown in formula (III); S5) the compound shown in the formula (III) is reacted with formic hydrazide to obtain the compound shown in formula (IV); S6) the compound shown in formula (IV) is obtained; The compound shown in the formula (IV) is reacted with the haloacetate shown in the formula (V) to obtain the compound shown in the formula (VI); S7) the compound shown in the formula (VI) is brominat...
Embodiment 1
[0062] Step 1: Add 50.0g cyclopropylnaphthalene and 300ml 1,2-dichloroethane into a 1L three-neck flask, stir to dissolve, add HNO dropwise 3 40.3g, after dropping, heated to reflux for 6h, added dropwise 50ml of ice water, stirred and separated, washed with 5% sodium bicarbonate solution 50ml×3, dried the organic phase with anhydrous sodium sulfate, filtered, concentrated to dryness The yellow oil mixture of formula (1) and formula (2) was directly put into the next step without treatment.
[0063] Step 2: Add the obtained light yellow oil, 150ml of purified water, 150ml of acetic acid, and 50.0g of iron powder into a 1L three-necked flask, heat to reflux for reaction until TLC shows that the reaction is complete (usually about 5 to 6 hours), and cool down to room temperature Filtrate, slowly pour the filtrate into 1L of ice water, stir, then extract with 500ml of dichloromethane × 3, combine the organic phases, wash with 300ml of saturated sodium bicarbonate × 2, wash once ...
Embodiment 2
[0071] Step 1: Same as Example 1.
[0072] Step 2: Add the obtained light yellow oil, 200ml of saturated ammonium chloride purified aqueous solution, and 48.0g of iron powder into a 1L three-necked flask, heat to reflux until TLC shows that the reaction is complete (usually about 5 to 6 hours), and cool down to Filtrate at room temperature, slowly pour the filtrate into 1L of ice water, stir, then extract with 500ml of dichloromethane × 3, combine the organic phases, wash with 300ml of saturated sodium bicarbonate × 2, wash once with 300ml of saturated saline, and dry over anhydrous sodium sulfate. Filter and concentrate to dryness to obtain a brownish-red oil, add 200ml of methanol and 46g of tartaric acid, slowly cool down to -10°C, crystallize for 4h, filter, wash with ice methanol, and dry under vacuum at 50°C to obtain 31.3g of a reddish-brown solid, which is the formula ( II) Crude product of the indicated compound.
[0073] Take 31.0g of the obtained reddish-brown soli...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com