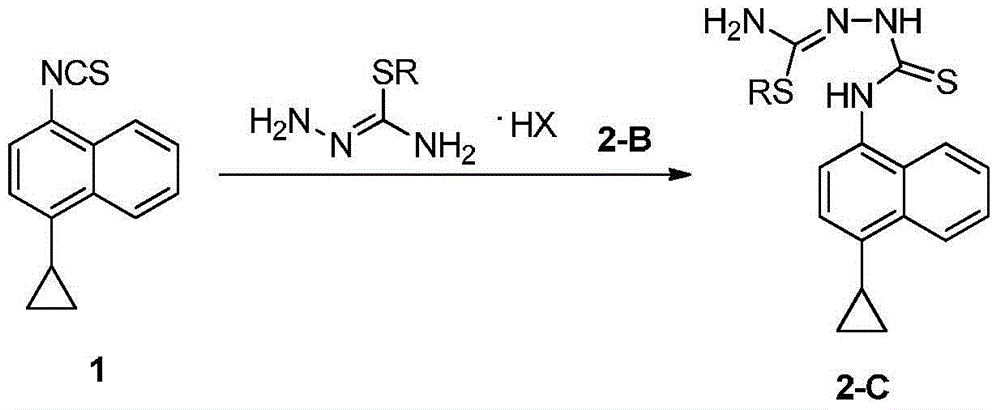
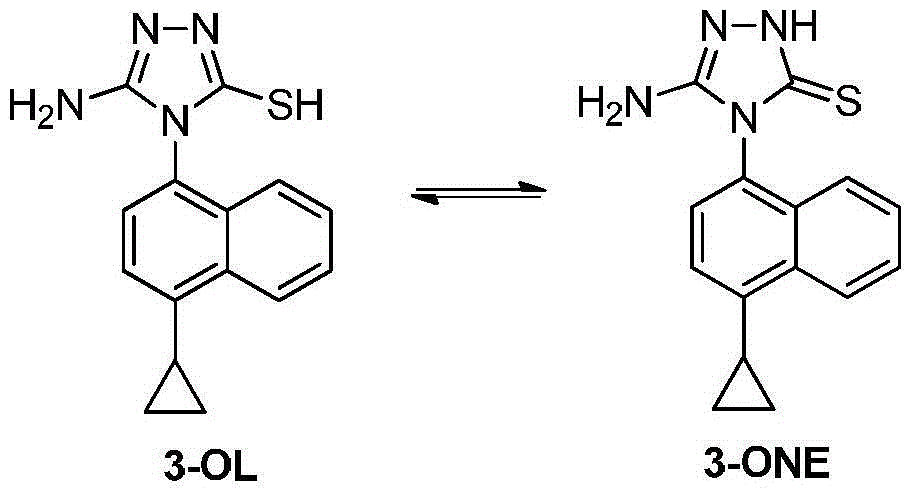
Preparation method of lesinurad intermediate namely 1-naphthyltriazole thioketone
A technology of thiosemicarbazide and C1-C3, which is applied in the field of medicine and can solve problems such as difficult operation, long reaction time, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]
[0025] A. Preparation of Compound 2-C2
[0026] Add 9.11g (0.1mol) thiosemicarbazide (2-A), 17.10g (0.1mol) benzyl bromide and 50mL absolute ethanol in a 500mL round bottom flask, then heat and reflux for 30min, at this time TLC analysis found that the reaction Finish.
[0027] The reaction compound was cooled to room temperature, and 200 mL of water was slowly added under stirring, followed by 22.53 g (0.1 mol) of compound 1 and a sodium carbonate solution prepared from 5.30 g (0.05 mol) of sodium carbonate and 25 mL of water. After the addition, the reaction mixture was continued to stir at room temperature for 10 hours to obtain a white slurry, the solid was collected by suction filtration, and then vacuum-dried at room temperature to obtain compound 2-C1, 32.53g, yield 80% (1→2) , ESI-MS, m / z=407 ([M+H] + ).
[0028] B. Preparation of compound 3
[0029] 28.46 g (0.07 mol) of the above-prepared compound 2-C1 and 300 mL of absolute ethanol were refluxed for ...
Embodiment 2
[0032] Add 9.11g (0.1mol) thiosemicarbazide (2-A), 17.10g (0.1mol) benzyl bromide and 50mL absolute ethanol in a 500mL round bottom flask, then heat and reflux for 30min, at this time TLC analysis found that the reaction Finish.
[0033] The reaction compound was cooled to room temperature, and 100 mL of water was slowly added with stirring, followed by the addition of 22.53 g (0.1 mol) of compound 1 and a sodium carbonate solution prepared from 6.91 g (0.05 mol) of potassium carbonate and 25 mL of water. After the addition was complete, the reaction mixture was stirred at 50°C for an additional 3 hours, at which time TLC indicated that the reaction was complete. The resulting reaction mixture was poured into 200 mL of ice water, stirred, extracted with 200 mL×4 ethyl acetate, combined organic phases, washed with 100 mL of brine, and dried over anhydrous sodium sulfate. The desiccant was removed by suction filtration, and the filtrate was evaporated to dryness on a rotary eva...
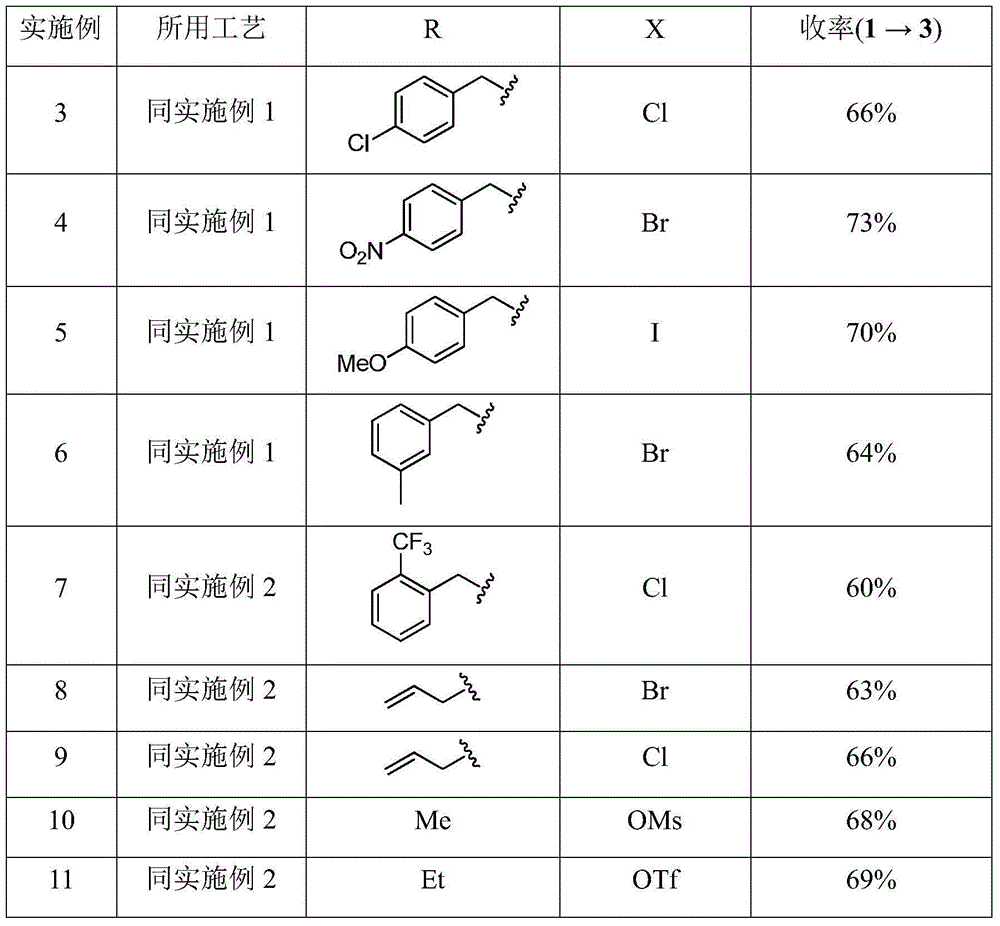
Embodiment 3-11
[0037] Adopt the technology of embodiment 1-2, change R and X, also can realize the object of the present invention, as shown in the table below.
[0038]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com