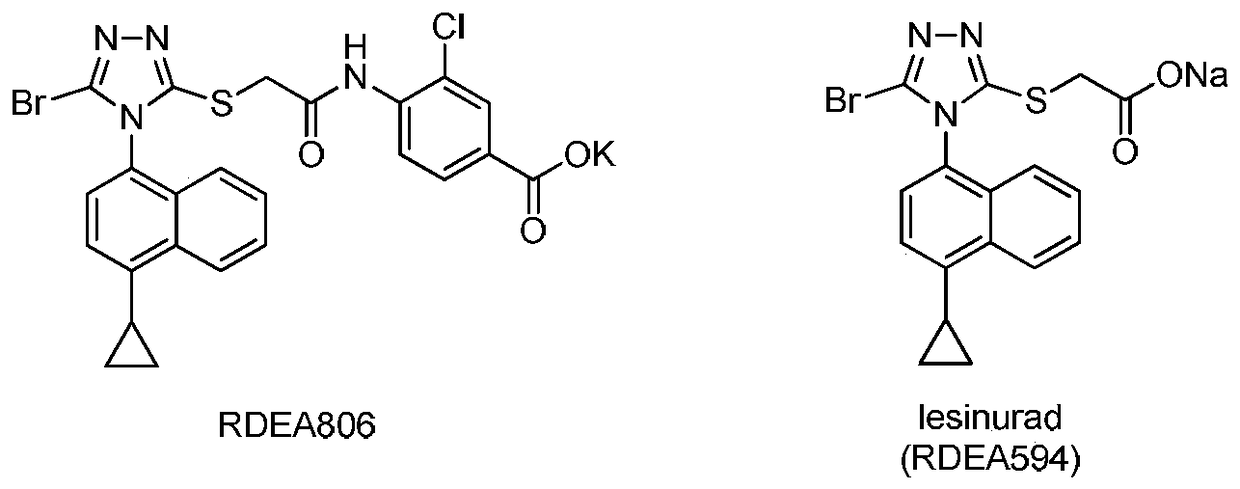
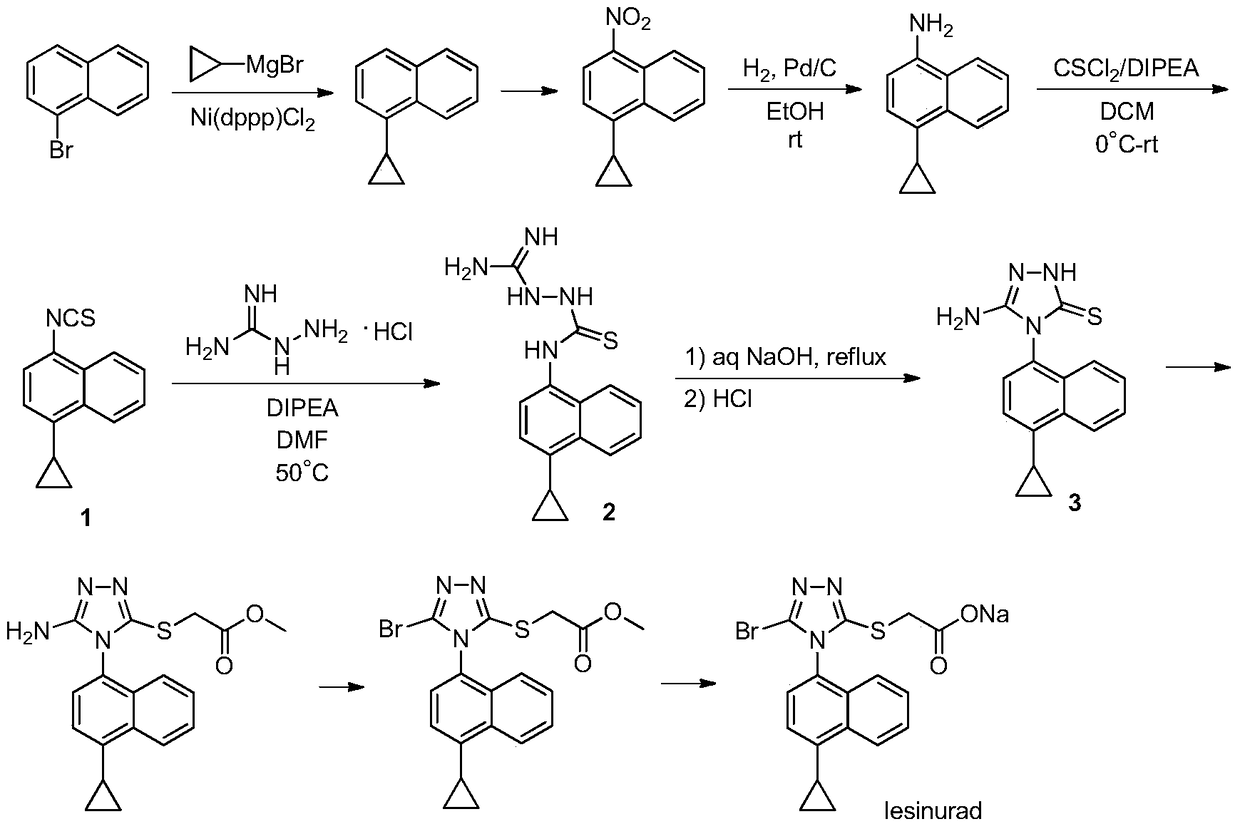
A process for refining the lesinurad intermediate 1-naphthyltriazolethione that can be industrialized
A process and compound technology, applied in the field of medicine, can solve the problems of hindering use, difficult to remove, difficult to smoothly perform stirring, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Pilot scale-up based on the process route in CN201410340622.9
[0025]
[0026] Step 1. Synthesis of compound 2-C1
[0027] Add 182.28g (2mol) of thiosemicarbazide 2-A into a 20L glass reactor, dissolve in 2097mL of absolute ethanol, add 342.06g (2mol) of benzyl bromide dropwise under cooling in an ice-water bath, and finish dropping within 10 minutes. After the dropwise addition, the temperature of the reaction mixture was raised to reflux for 30 minutes. TLC inspection found that the reaction was complete, and then the reaction mixture was cooled to room temperature, and the ethanol solution of 2-B1 was obtained at this time.
[0028] 8388mL of water and 450.62g (2mol) of compound 1 were added successively to the reaction mixture, and then a saturated solution of sodium carbonate prepared by 105.99g (1mol) of sodium carbonate and 321mL of water was added under stirring. After the addition, the reaction mixture was mechanically stirred at room temperatu...
Embodiment 2
[0038] Embodiment 2 The improved technique of the present invention
[0039]
[0040] Step 1. Synthesis of compound 2-C1
[0041] Add 182.28g (2mol) of thiosemicarbazide 2-A to a 10L round bottom flask, dissolve it in 2097mL of absolute ethanol, add 342.06g (2mol) of benzyl bromide dropwise under cooling in an ice-water bath, and finish dropping within 10 minutes. After the dropwise addition, the temperature of the reaction mixture was raised to reflux for 30 minutes. TLC inspection found that the reaction was complete, and then the reaction mixture was cooled to room temperature, and the ethanol solution of 2-B1 was obtained at this time.
[0042] To the reaction mixture, 2097mL of water and 450.62g (2mol) of compound 1 were added successively, and then a saturated solution of sodium carbonate prepared by 105.99g (1mol) of sodium carbonate and 321mL of water was slowly added dropwise under stirring, and the dropping time continued for 4 hours. After the dropwise additio...
Embodiment 3
[0049] Embodiment 3 The improved technique of the present invention
[0050] Step 1. Synthesis of compound 2-C1
[0051] Add 182.28g (2mol) of thiosemicarbazide 2-A to a 10L round bottom flask, dissolve it in 2097mL of absolute ethanol, add 342.06g (2mol) of benzyl bromide dropwise under cooling in an ice-water bath, and finish dropping within 10 minutes. After the dropwise addition, the temperature of the reaction mixture was raised to reflux for 30 minutes. TLC inspection found that the reaction was complete, and then the reaction mixture was cooled to room temperature, and the ethanol solution of 2-B1 was obtained at this time.
[0052] 2097mL of water and 450.62g (2mol) of compound 1 were added successively to the reaction mixture, and then a saturated solution of sodium carbonate prepared by 105.99g (1mol) of sodium carbonate and 321mL of water was slowly added dropwise under stirring for 2 hours. After the dropwise addition, the reaction mixture was stirred overnight ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com