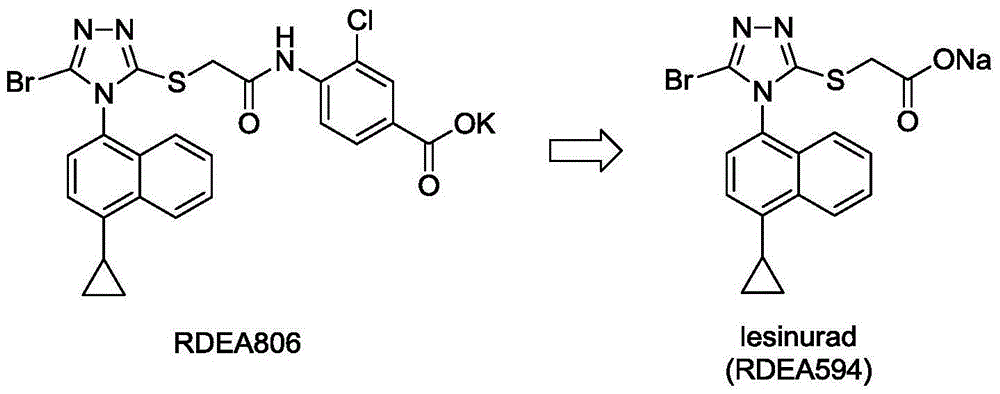
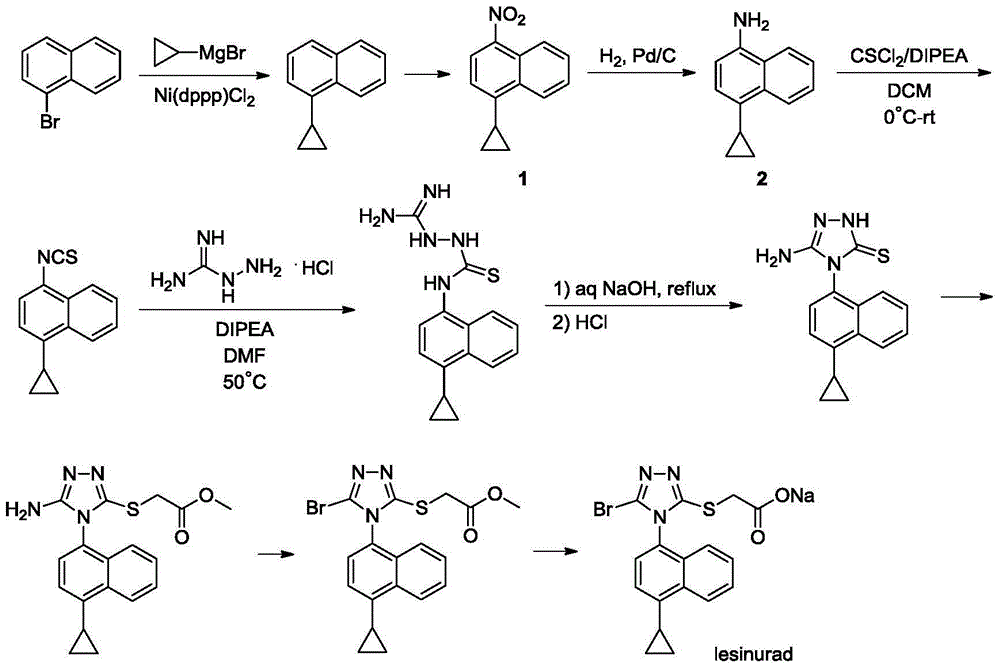
Method for producing lesinurad intermediate 4-cyclopropyl-1-naphthylamine
A compound and molar technology, applied in the field of medicine, can solve problems such as unsatisfactory safety and economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
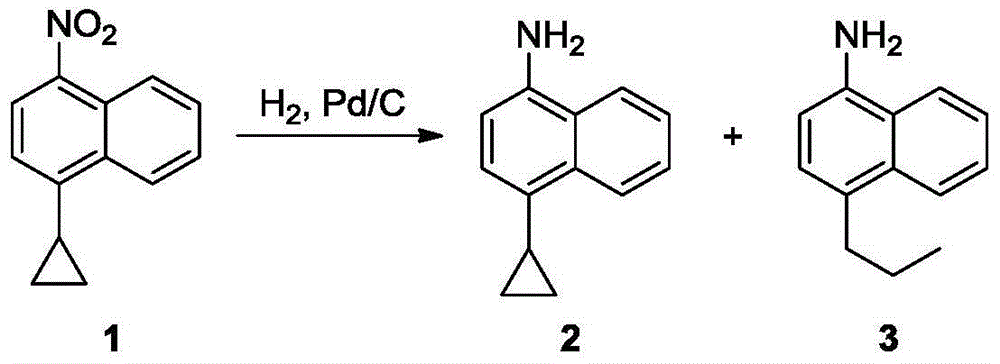
[0014] 21.32g (0.1mol) of 4-cyclopropyl-1-nitronaphthalene 1 was dissolved in 300mL of absolute ethanol, stirred at room temperature, 7.68g of 10% Pd / C was added, and catalytic hydrogenation was carried out overnight at room temperature, TLC showed that the reaction was complete .
[0015] The reaction mixture was suction filtered to remove the catalyst, the filtrate was evaporated on a rotary evaporator to remove the solvent, and the obtained residue was purified by column chromatography to obtain product 2, a light red oily liquid, 13.56 g, with a yield of 74%. 1 HNMR (DMSO-d 6 ,400MHz),δ:8.24(d,1H,J=8.4Hz),8.07(d,1H,J=8.4Hz),7.46-7.50(m,1H),7.37-7.41(m,1H),6.99( d,1H,J=7.6Hz),6.59(d,1H,J=8.0Hz),5.52(bs,2H),2.10-2.15(m,1H),0.89-0.93(m,2H),0.53-0.57 (m,2H).
[0016] go through 1 According to HNMR analysis, the compound 2 prepared above contains a certain amount of by-product 3 (see summary in Table 1).
Embodiment 2
[0018] 21.32g (0.1mol) of 4-cyclopropyl-1-nitronaphthalene 1 was dissolved in 1000mL of absolute ethanol, stirred at room temperature, 7.68g of 10% Pd / C was added, and catalytic hydrogenation was carried out overnight at room temperature, TLC showed that the reaction was complete .
[0019] The reaction mixture was suction filtered to remove the catalyst, the filtrate was evaporated to remove the solvent on a rotary evaporator, and the obtained residue was purified by column chromatography to obtain product 2, a light red oily liquid, 13.38 g, with a yield of 73%.
[0020] go through 1 According to HNMR analysis, the compound 2 prepared above contains a certain amount of by-product 3.
Embodiment 3
[0022] 21.32g (0.1mol) of 4-cyclopropyl-1-nitronaphthalene 1 was dissolved in 200mL of absolute ethanol, stirred at room temperature, 1.07g of 10% Pd / C was added, and catalytic hydrogenation was carried out overnight at room temperature, TLC showed that the reaction was complete .
[0023] The reaction mixture was suction filtered to remove the catalyst, the filtrate was evaporated to remove the solvent on a rotary evaporator, and the obtained residue was purified by column chromatography to obtain product 2, a light red oily liquid, 13.01 g, with a yield of 71%.
[0024] go through 1 According to HNMR analysis, the compound 2 prepared above contains a certain amount of by-product 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com