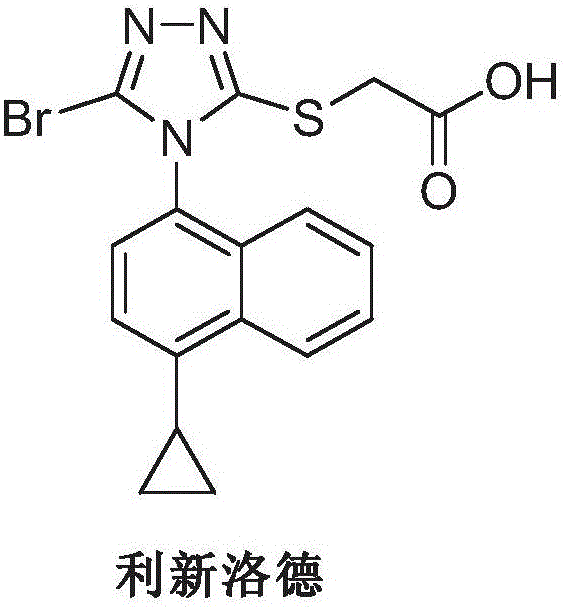
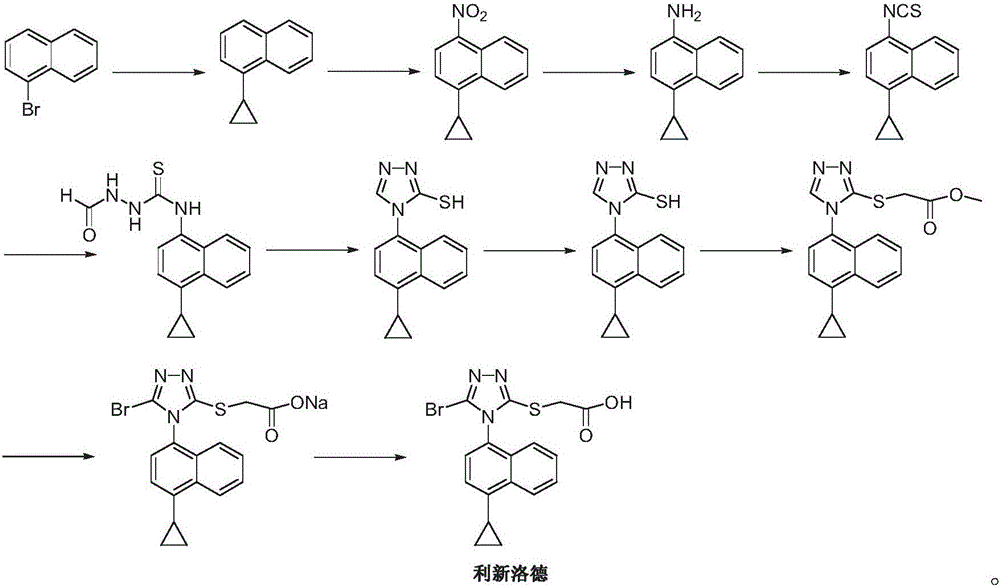
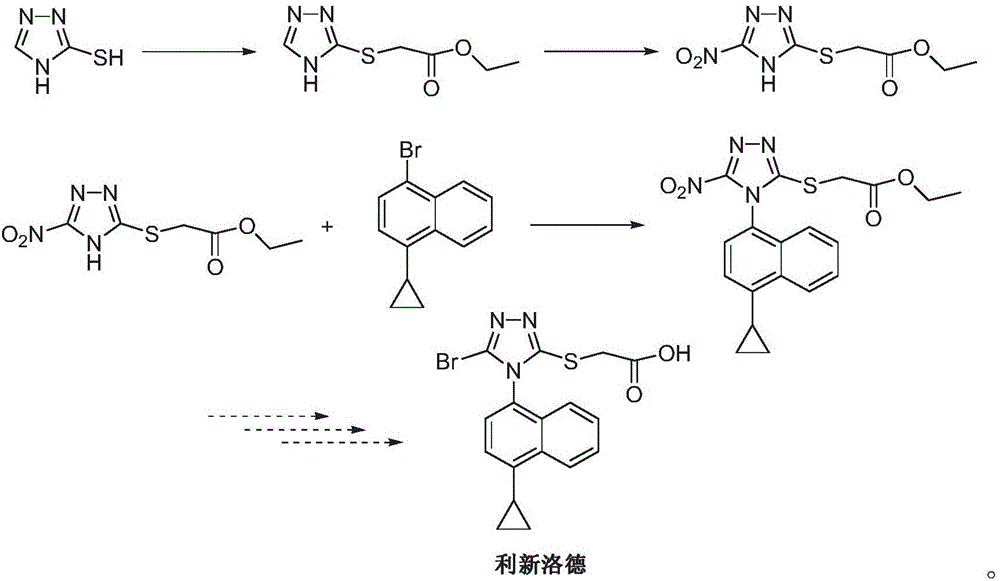
Preparation method of Lesinurad
A technology of condensation reaction and raw materials, applied in the field of preparation of new drug Lixinlord, can solve the problems of low total yield, difficult industrialization, many reaction steps, etc., and achieves the effect of environmental protection and economy, simple process, and promotion of development.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1-bromo-4-cyclopropylnaphthalene (II) (12.4g, 0.05mol), 2-[(3-bromo-4H-1,2,4-triazol-5-yl)thio]acetic acid ( III) (14.3g, 0.06mol), cuprous iodide (1.42g, 7.5mmol), 8-hydroxyquinoline (2.2g, 7.5mmol), potassium carbonate 7.6g (0.055mol) and 100mL N, N-di Add methylformamide into a 250mL three-necked flask, raise the temperature to 100°C, and stir until dissolved.
[0033] Add ethylenediamine (0.45g, 7.5mmol), continue to heat up to 120 degrees, react for 5-7 hours, TLC detection, the reaction is complete. Cool down to 50-60 degrees, filter, and wash the filter cake with ethyl acetate. The organic phases were combined, washed with brine and water, concentrated, and slurried with ethyl acetate and water (9:1) to obtain 14.6 g of beige solid Lixinluode (I), with a yield of 72.3%. ESI-MS (m / z): 405 (M+H), 1 H NMR (400MHz, CDCl3) 12.92 (brs, 1H), 8.57 (d, J = 8.5Hz, 1H), 7.65 (m, 3H), 7.41 (d, J = 7.5Hz, 1H), 7.14 (d, J =7.8Hz, 1H), 3.97(s, 2H), 2.52(m, 1H), 1.14(m.2H), ...
Embodiment 2
[0035] 1-bromo-4-cyclopropylnaphthalene (II) (12.4g, 0.05mol), 2-[(3-bromo-4H-1,2,4-triazol-5-yl)thio]acetic acid ( III) (14.3g, 0.06mol), cuprous iodide (1.42g, 7.5mmol), 4-dimethylaminopyridine (0.9g, 7.5mmol), potassium tert-butoxide 6.2g (0.055mol) and 100mL dimethyl Add sulfoxide into a 250mL three-necked flask, raise the temperature to 100°C, and stir until dissolved.
[0036] Add diisopropylethylamine (1.0 g, 7.5 mmol), continue to heat up to 130 degrees, and react for 6 to 8 hours. TLC detects that the reaction is complete. Cool down to 50-60 degrees, filter, and wash the filter cake with ethyl acetate. The organic phases were combined, washed with brine and water, concentrated, and slurried with ethyl acetate and water (9:1) to obtain 13.5 g of beige solid Lixinluode (I), with a yield of 66.8%.
Embodiment 3
[0038] 2-[(3-amino-4H-1,2,4-triazol-5-yl)thio]acetic acid (IV) (8.7g, 0.05mol) and 48% concentration of hydrobromic acid (15mL, 0.125 mol) into a 100mL single-port reaction flask, lower the temperature to 0°C, add dropwise a 10mL aqueous solution of sodium nitrite (3.5g, 0.05mol) under stirring, keep the temperature between 5°C and 10°C, and drop it in about 30 minutes. spare.
[0039] Add cuprous bromide (3.9g, 27.5mmol) and 48% concentration of hydrobromic acid (4mL, 0.03mol) in a 100mL three-necked flask, stir and dissolve, place in an ice bath, add the above-mentioned standby solution dropwise, and keep The temperature is 5-10 degrees. After dropping, raise the temperature to room temperature and continue to stir and react for 30 minutes, then continue to slowly raise the temperature to 50-60 degrees, stir and react until no gas overflows, and stop the reaction. Stand still, filter, and recrystallize the filter cake with ethanol and water (volume ratio 1:1) to obtain off...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com