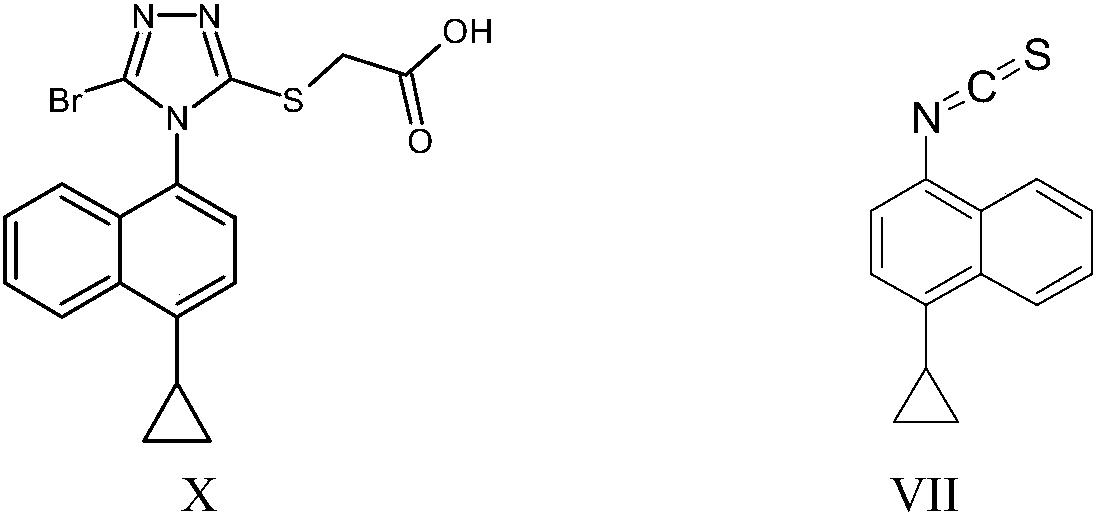
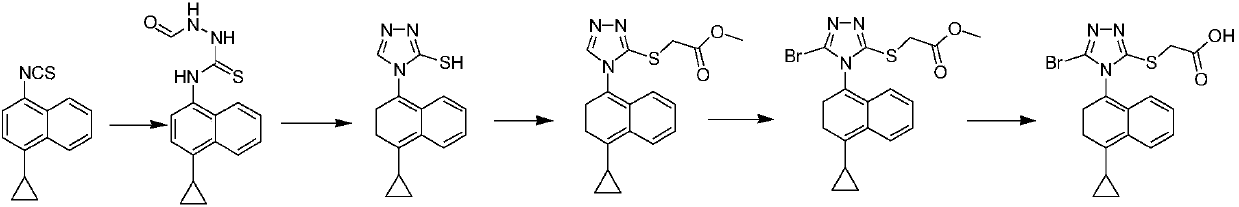
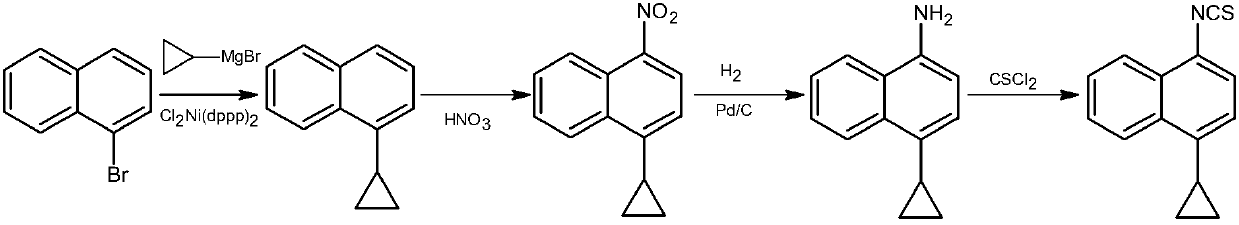
Naphthyl thiocarbonyl compound as well as preparation method and application thereof
A technology of naphthylthiocarbonyl and compound, which is applied in the application field of synthesizing racinades, can solve the problems of high risk, high production cost, reduced product purity and yield, etc., and achieves high product purity and low pollution , the effect of low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Example 1 Preparation of 1-cyclopropylnaphthalene (III)
[0101] Under nitrogen protection, into a dry 2L round bottom flask, add 1-bromonaphthalene (50 g, 241 mmol), ferric chloride (2.0 g, 12.3 mmol) and 100 mL of dry tetrahydrofuran (THF), and stir under ice-water bath cooling, Using a constant pressure dropping funnel, 300 mL of a 1.0 mol / L cyclopropylmagnesium bromide solution in THF was slowly added dropwise to the system. After the dropwise addition, the reaction compound was stirred at room temperature for 5 h under nitrogen protection, then heated to reflux, and reacted for 24 h. After the reaction was completed, most of the solvent was distilled off under reduced pressure, the residue was cooled to room temperature, slowly poured into 2L of stirred ice water, stirred, adjusted to pH 2-3 with concentrated hydrochloric acid, and extracted with dichloromethane (250mL×3) . The organic phases were combined and washed with 500 mL of water. Evaporate to dryness ...
Embodiment 2
[0102] Example 2 Preparation of 1-cyclopropylnaphthalene (III)
[0103] Under nitrogen protection, into a dry 2L round-bottom flask, add 1-bromonaphthalene (50 g, 241 mmol), bistriphenylphosphine nickel dichloride (2.5 g, 4.6 mmol) and 100 mL of dry THF, and cool in an ice-water bath While stirring, 300 mL of a 1.0 mol / L cyclopropylmagnesium bromide solution in THF was slowly added dropwise to the system using a constant pressure dropping funnel. After the dropwise addition, the reaction compound was stirred at room temperature for 5 h under nitrogen protection, then heated to reflux, and reacted for 24 h. After the completion of the reaction, most of the solvent was distilled off under reduced pressure, the residue was cooled to room temperature, slowly poured into 2L of stirred ice water, stirred, adjusted to pH 2-3 with concentrated hydrochloric acid, extracted with dichloromethane (250mL×3) . The organic phases were combined and washed with 500 mL of water. Evaporate...
Embodiment 3
[0104] Example 3 Preparation of 4-cyclopropyl-1-bromonaphthalene (IV)
[0105] Put 300 mL of dichloromethane, 1-cyclopropylnaphthalene (30 g, 178 mmol), and dibromohydantoin (50.9 g, 178 mmol) into a dry reaction flask, control the internal temperature to 30-50 ° C, and stir the reaction until TLC detects that the reaction is complete , stop keeping warm. Add 100 mL of sodium hydrogen sulfite aqueous solution (5%) to wash, 100 mL of sodium hydroxide solution (5%) to wash, and wash with water until neutral. Evaporate to dryness under reduced pressure to obtain 4-cyclopropyl-1-bromonaphthalene (39.8 g, yield 90.48%, purity 97.6%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com