New compound for gout and preparation method thereof, and application and pharmaceutical preparation of new compound
A compound and chemical technology, applied in the field of medicine, can solve the problem of inability to quickly oxidize and eliminate uric acid, and achieve the effect of simple preparation method and lower blood uric acid concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
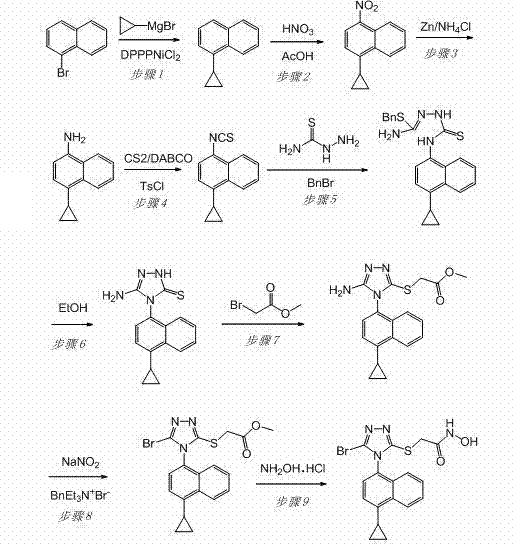
[0034] Example 1: Preparation of 2-[5-bromo-4-(4-cyclopropyl-1-naphthyl)-4H-1,2,4-triazole-3-sulfanyl]-N-hydroxyacetamide
[0035] The technical route is as follows:
[0036]
[0037] 1) Preparation of 1-cyclopropylnaphthalene
[0038] To a dry 5 L round bottom flask, add 1-bromonaphthalene (414.0 g, 2 mol), [1,3-bis(diphenylphosphino)propane]nickel chloride (165 g, 0.4 mol) and 1500 mL of dry 3000 mL of THF solution of 1.0 mol / L cyclopropylmagnesium bromide was slowly added dropwise to the system with a constant pressure dropping funnel. After the dropwise addition, the reaction compound was stirred at room temperature for 8 h under nitrogen protection, then heated to reflux, and reacted for 36 h. After the reaction was complete, the reaction mixture was cooled to room temperature, then carefully poured into 10 L of stirred ice water, stirred, and adjusted to pH 2 with concentrated hydrochloric acid, CH 2 Cl 2 (4000 mL×3) extraction. Combine the organic phases, wash...
Embodiment 2
[0057] Example 2: Preparation of formula 1 compound tablet
[0058] prescription:
[0059]
[0060] Preparation Process:
[0061] ①. Pretreatment: The compound of formula 1, lactose monohydrate, microcrystalline cellulose, croscarmellose, low-substituted hydroxypropyl cellulose and magnesium stearate were respectively pulverized through a 100-mesh sieve.
[0062] ②. Total mixing: mix the compound of formula 1 and lactose monohydrate, then add magnesium stearate to increase fluidity, and mix well
[0063] Add other ingredients and mix well.
[0064] ③, tableting: direct tableting, the temperature between tableting is 20-25℃, and the relative humidity is controlled at 35-45%.
Embodiment 3
[0065] Example 3: Bioactivity assay
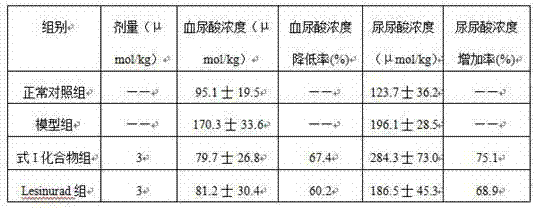
[0066] 1. Effect of formula I compound on mouse hyperuricemia
[0067] Experimental animals: healthy Kunming mice, weighing 15-20 g, provided by the Experimental Animal Center of Kunming Medical College. Breeding conditions: Room temperature is 25±2°C, relative humidity is 45-75%.
[0068] Grouping, modeling and administration: 40 Kunming mice were randomly divided into 4 groups, 10 in each group, half male and half male: normal control group, hyperuricemia model group, compound group of formula I, lesinurad group. The mice in the normal control group were injected intraperitoneally with 0.5% CMC-Na solution, and the mice in the other groups were injected intraperitoneally with oxonic acid potassium salt 500 mg / kg. After 1 hour, the mice in the normal control group and hyperuricemia model group were injected intraperitoneally. Volume 0.5% CMC-Na solution, and the mice in other groups were intraperitoneally injected with the test drug. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com