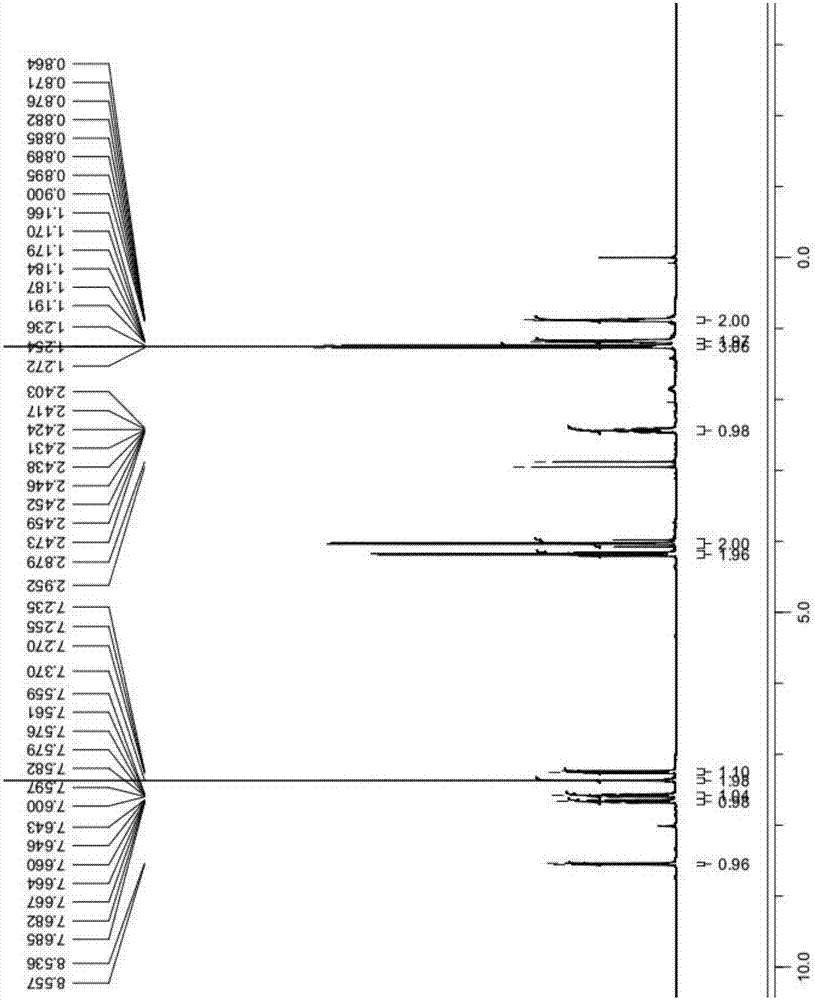
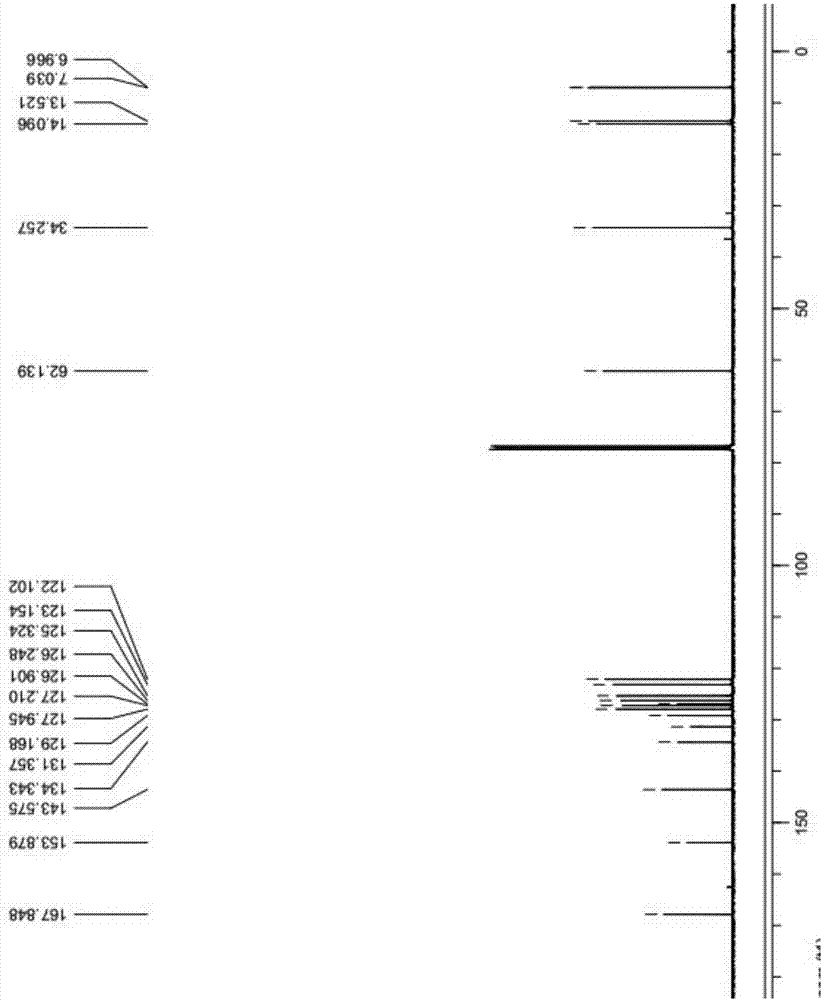
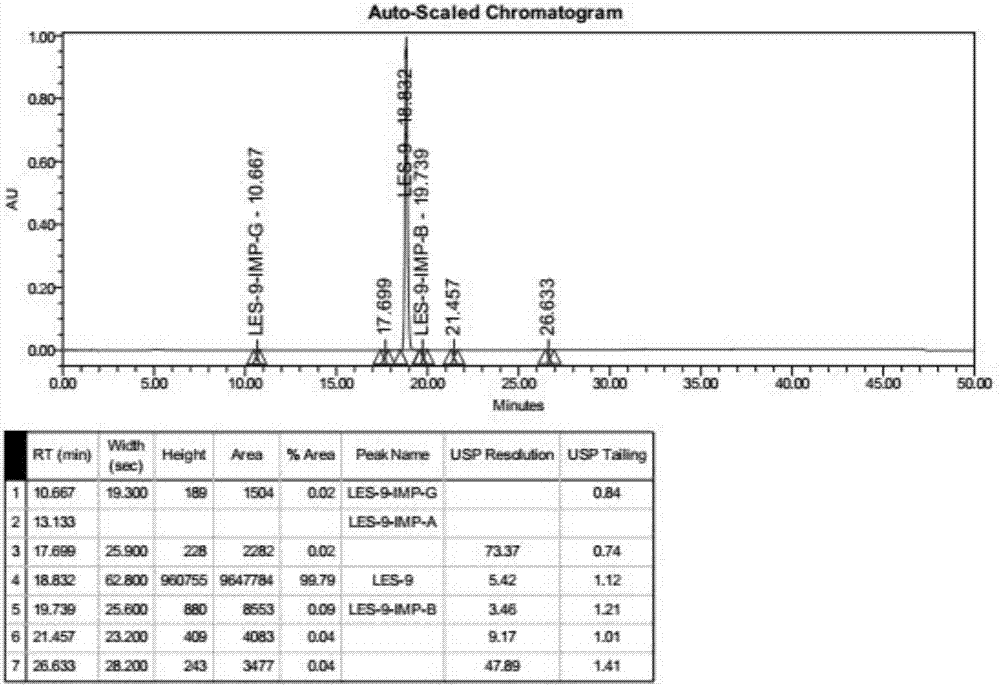
Synthetic method of Lesinurad
A synthesis method and compound technology, applied in the direction of organic chemistry, can solve the problems of unfavorable industrialization, etc., and achieve the effect of less side reactions, simple and easy preparation method, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The synthetic method of Lesinurad in the present embodiment comprises the following steps:
[0043] (1) At room temperature, mix 100g of the compound of formula 7, 500mL of DMF, and 48g of methyl carbazate into the reaction flask, heat the reaction mixture to 50-60°C, and keep stirring for 5-8 hours; the reaction is over Afterwards, the reaction solution was cooled to room temperature, and 4000 mL of water was added to precipitate the product, filtered and dried to obtain 125 g of the dry product of the compound of formula 6, with a yield of 89%;
[0044] (2) Add 100g of the compound of formula 6 to 1500mL of 1M sodium hydroxide solution at room temperature, heat to 80-90°C for 1 hour to dissolve, cool to room temperature after TLC detection, add dropwise 3500mL of 5% hydrochloric acid solution to precipitate The solid was filtered to obtain a wet product, and dried in a hot air oven at 55°C to constant weight to obtain 85 g of the dry product of the compound of formula...
Embodiment 2
[0049] (1) At room temperature, mix 50g of the compound of formula 7, 300mL of DMF, and 24g of methyl carbazate and add them to the reaction flask, heat the reaction mixture to 50-60°C, and keep stirring for 7 hours; The reaction solution was cooled to room temperature, and 2500 mL of water was added to precipitate the product, filtered and dried to obtain 60 g of the compound of formula 6 as a dry product, with a yield of 88%;
[0050] (2) Add 50g of the compound of formula 6 to 700mL of 1M sodium hydroxide solution at room temperature, heat to 80-90°C for 1 hour to dissolve, cool to room temperature after TLC detection, add dropwise 1750mL of 5% hydrochloric acid solution to precipitate The solid was filtered to obtain a wet product, and dried in a hot air oven at 55°C to constant weight to obtain 42 g of the dry product of the compound of formula 5, with a yield of 95%;
[0051] (3) At room temperature, mix 0.1 mol of the compound of formula 5 with potassium carbonate and D...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com