Preparation method of Lesinurad
A compound, selected technology, applied in chemical instruments and methods, compounds containing periodic table Group 3/13 elements, bulk chemical production, etc. problem, to achieve the effect of low cost, high reliability and short process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
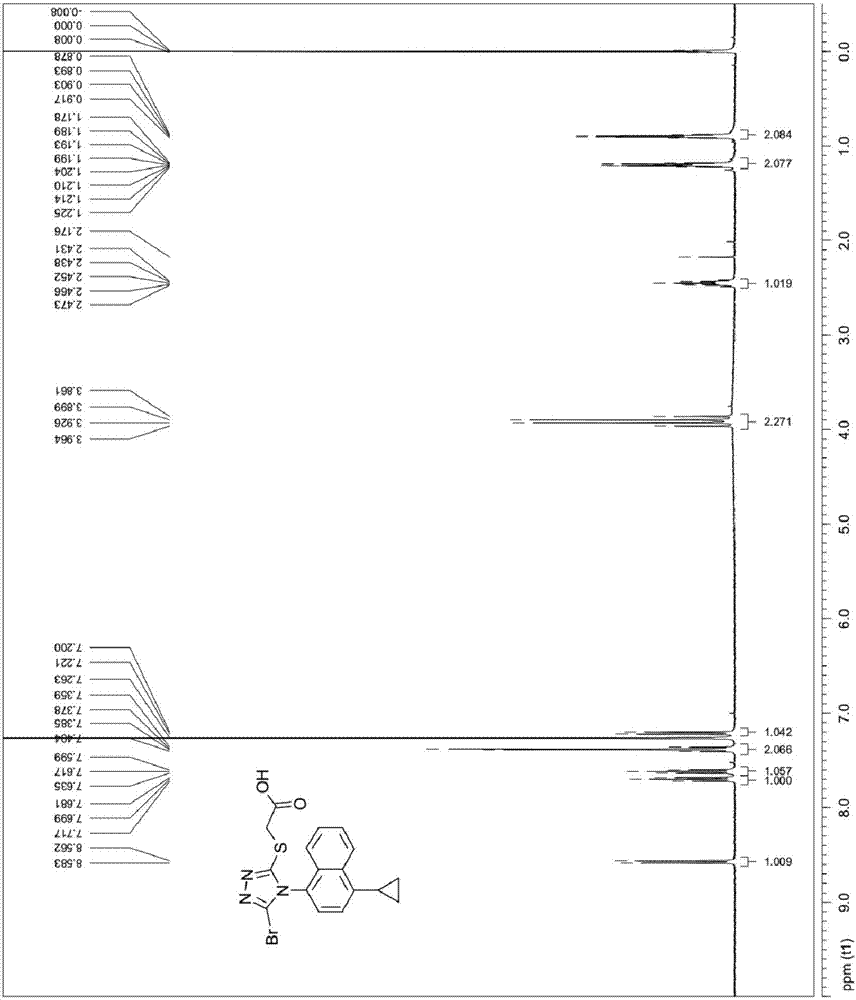
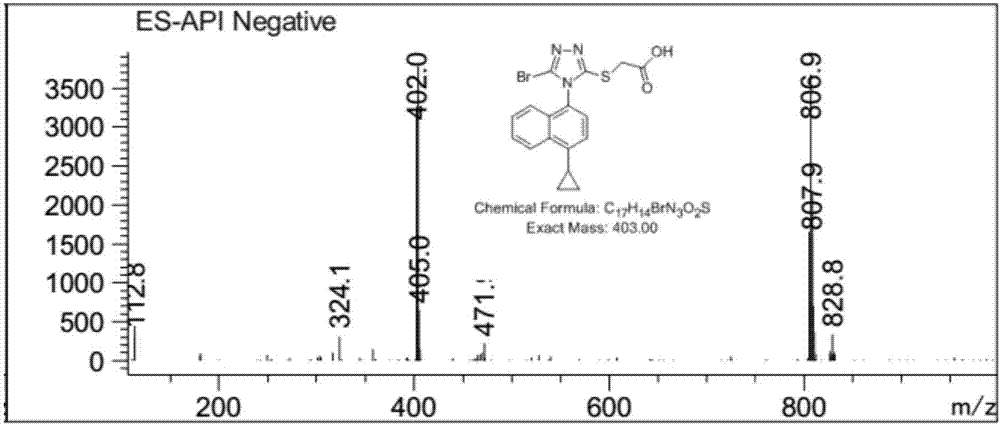
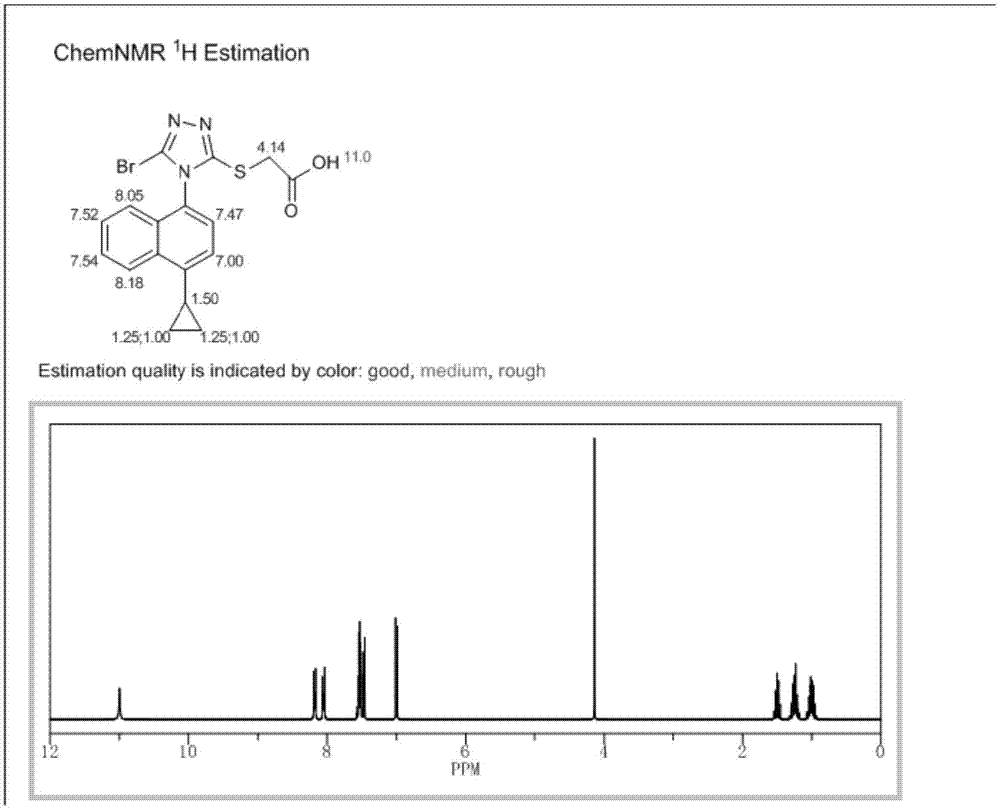
[0051] The synthetic method of embodiment 1 Resinard
[0052] (1) Synthesis of Les-03
[0053]
[0054] Put 572mg of 1,4-dibromo-naphthalene in a 100mL flask, add 20mL of dioxane and 20mL of 2mol / L potassium carbonate solution, stir well, then continue to add 176mg of cyclopropylboronic acid and 100mg of tetrakis(triphenyl Phosphine) palladium, then pass through nitrogen exchange for 10 minutes, under the protection of nitrogen, heat and reflux at 100°C for 4 hours, HPLC detection reaction, after the reaction is completed, add 20mL ethyl acetate to the reaction solution for extraction, continuous extraction for 2 Then add saturated brine to wash twice, 20 mL each time, combine the organic phases, concentrate under reduced pressure, and filter to obtain 430 mg of 1-bromo-4-cyclopropyl-naphthalene, with a yield of 87% and a purity of 96.5%.
[0055] (2) Synthesis of Les-05
[0056]
[0057] Put 250mg of 1-bromo-4-cyclopropyl-naphthalene in a 100mL flask, add 5mL of dioxa...
Embodiment 2
[0067] The synthetic method of embodiment 2 Resinard
[0068] (1) Synthesis of Les-03
[0069]
[0070] Put 28.6g of 1,4-dibromo-naphthalene in a 10L flask, add 1L of dioxane and 1L of 2mol / L sodium carbonate solution, stir well, then continue to add 17.6g of cyclopropylboronic acid and 7.5g Tetrakis(triphenylphosphine) palladium, then pass through nitrogen exchange for 10 minutes, under the protection of nitrogen, heat and reflux at 100°C for 5 hours, HPLC detects the reaction, after the reaction is completed, add 2L ethyl acetate to the reactant for extraction , continuously extracted 2 times, then added saturated brine to wash 2 times, 2L each time, combined the organic phases, concentrated under reduced pressure, and filtered to obtain 18.8g of 1-bromo-4-cyclopropyl-naphthalene, with a yield of 76.4%; purity was 96.1%.
[0071] (2) Synthesis of Les-05
[0072]
[0073] Place 12.5g of 1-bromo-4-cyclopropyl-naphthalene in a 10L flask, add 0.5L of dioxane solvent to ...
Embodiment 3
[0083] The synthetic method of embodiment 3 Resinard
[0084] (1) Synthesis of Les-03
[0085]
[0086] Place 286g of 1,4-dibromo-naphthalene in a 100L flask, add 10L of tetrahydrofuran and 10L of triethylamine, stir well, then continue to add 176g of cyclopropylboronic acid and 75g of tetrakis(triphenylphosphine)palladium, Then pass through nitrogen exchange for 15 minutes, under the protection of nitrogen, heat and reflux at 100°C for 5 hours, HPLC detects the reaction, after the reaction is completed, add 20L ethyl acetate to the reactant for extraction, continuous extraction 2 times, and then add saturated Wash with brine twice, 20 L each time, combine the organic phases, concentrate under reduced pressure, and filter to obtain 185 g of 1-bromo-4-cyclopropyl-naphthalene, with a yield of 75.2% and a purity of 95.6%.
[0087] (2) Synthesis of Les-05
[0088]
[0089] Place 125g of 1-bromo-4-cyclopropyl-naphthalene in a 100L flask, add 0.5L tetrahydrofuran solvent to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com