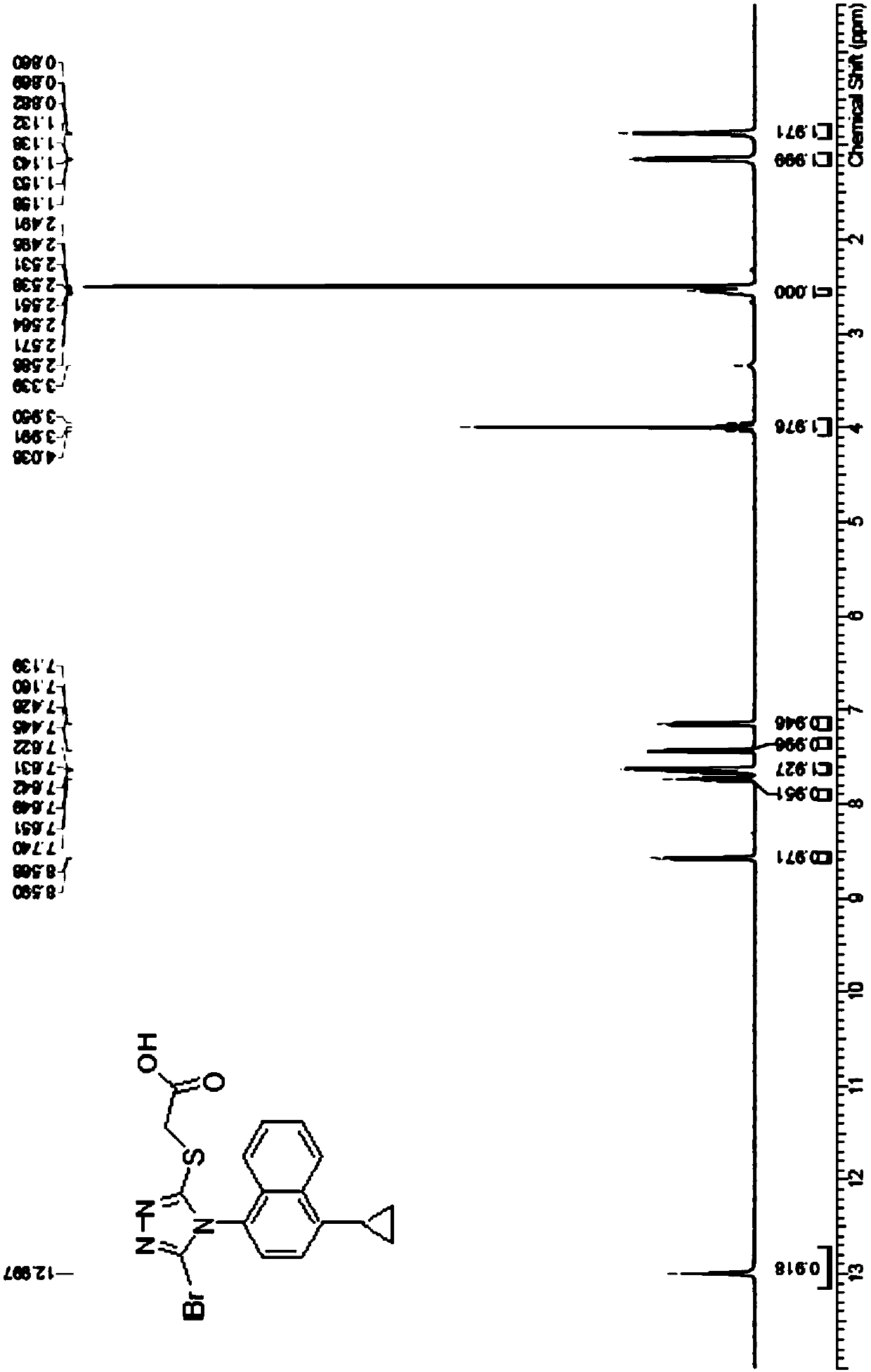
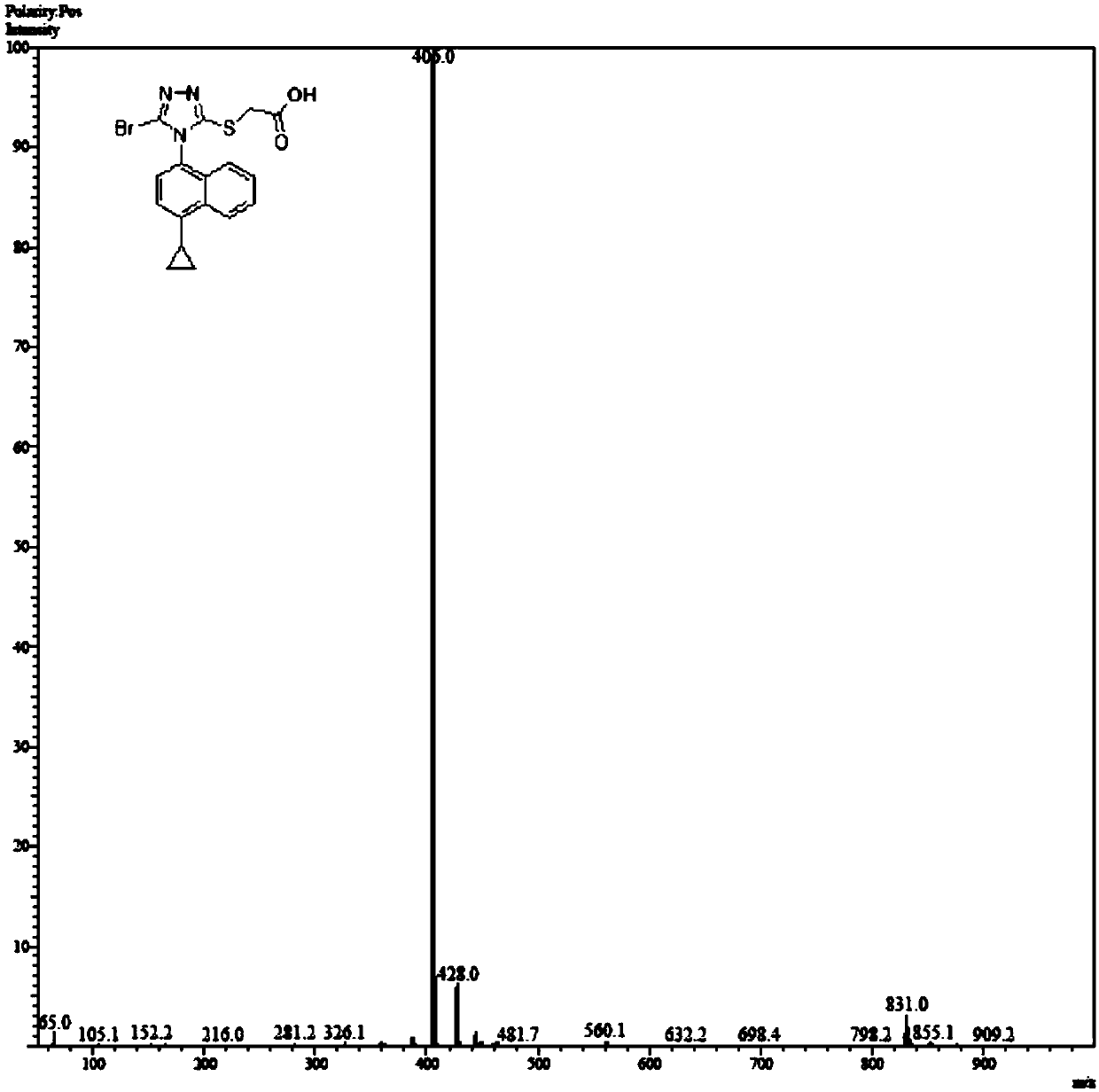
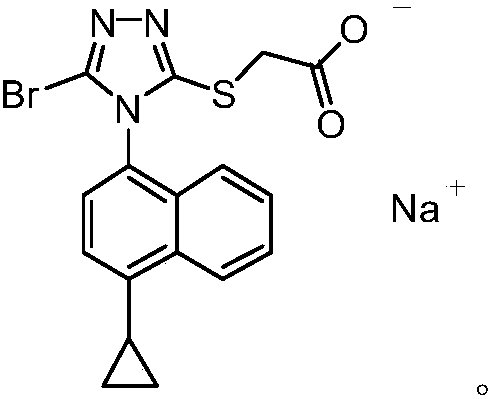
Refining method of lesinurad
A refining method and high-quality technology, applied in the field of medicine, can solve the problems of tediousness and incompatibility with the requirements of industrialized large-scale production, and achieve the effects of easy drying and shortening of drying time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1) Add 400ml of the reaction solvent DMF to a 1000ml three-necked reaction flask, and then add 100g of starting material 4-(4-cyclopropylnaphthalene-1-yl)-1H-1,2,4-tri Azole-5(4H)-thiol was stirred and dissolved at 15-20°C to obtain A solution. Add 39.7g of powdered anhydrous sodium carbonate to solution A, and add 65.7g of ethyl bromoacetate dropwise at 10-15°C to control the rate of addition. During the dropping process, the internal temperature does not exceed 15°C. The internal temperature was 10-15°C, and the reaction was carried out with heat preservation. After thin-layer chromatography, the reaction of the raw materials was complete, and the reaction was stopped after extending the reaction for 15 minutes.
[0037] Transfer the reaction solution to a 5L beaker, add 1.2L of purified water at 0-5°C dropwise to the system at an internal temperature of 0-5°C, a large amount of precipitation occurs, continue to stir at 0-5°C for 0.5h, and filter with suction. When a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com