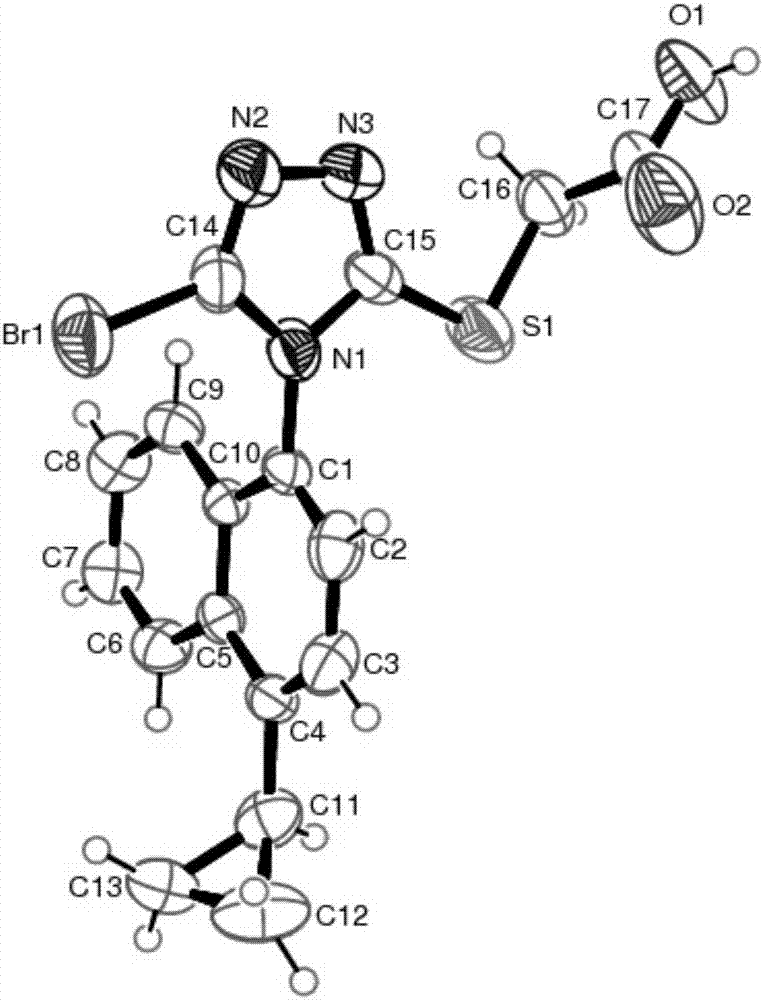
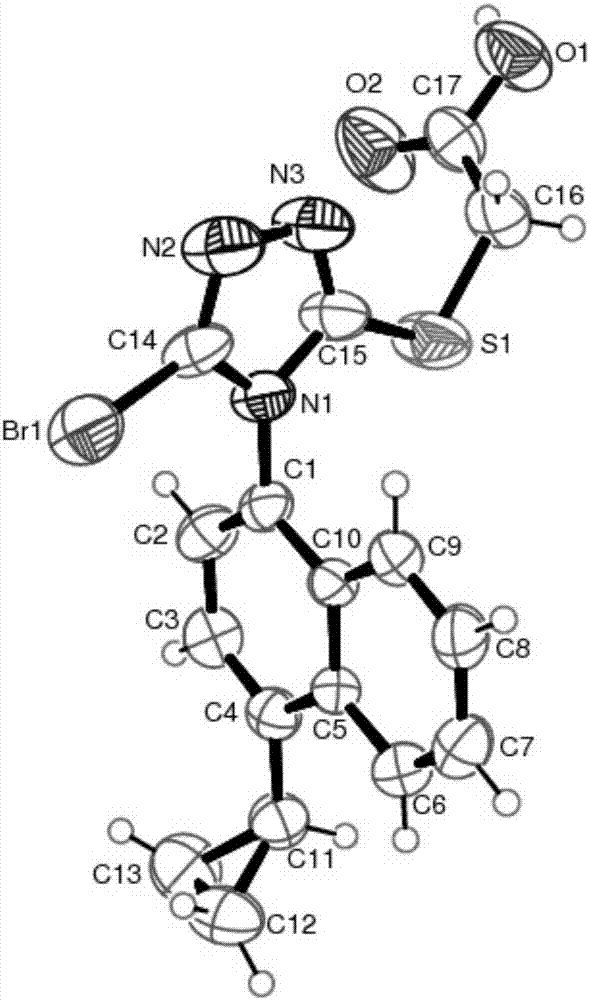
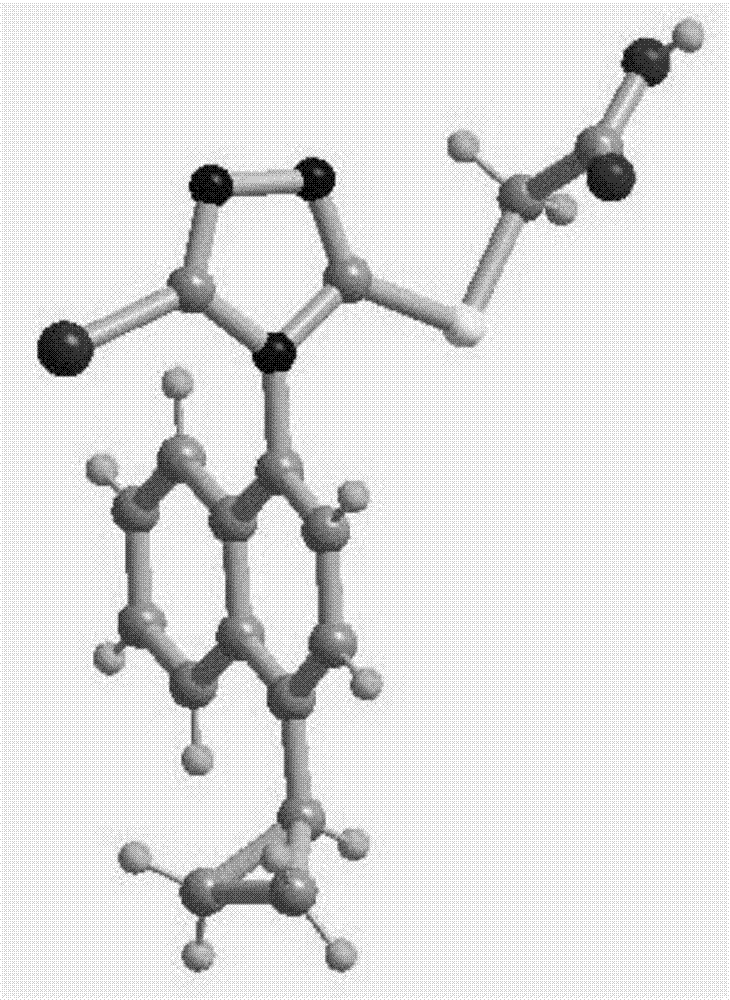
Resolving method of medicine lesinurad axial chiral enantiomer
An enantiomeric and axial chirality technology, applied in organic chemistry and other directions, can solve the problems of high cost, high resolution difficulty, and unsuitable for industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Preparation of racemic lesinurad
[0081] Referring to WO2014008295A1, lesinurad was synthesized. Proceed as follows:
[0082]
[0083] Add 4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazole-3-thiol (Compound A, 5g, 0.018mol), potassium carbonate (3.74 g, 0.027mol, 1.5eq), DMF (50mL). Ethyl bromoacetate (3.3 g, 0.022 mol, 1.2 eq) was added dropwise with stirring. After dropping, stir the reaction at room temperature for 1.5h. After the reaction of raw materials was detected by sampling, 100 mL of ice water was dripped into the reaction solution under stirring, and a white solid was precipitated. Stir for 30min, filter with suction and wash with water. After the filter cake was dried, it was recrystallized with ethyl acetate to obtain 4 g of white solid, namely Intermediate B.
[0084] 1 HNMR (CDCl 3 ): δ8.54(dd, J=8Hz, 1H), 8.31(S, 1H), 7.66(m, 1H), 7.57(m, 1H), 7.40(dd, J=8Hz, 1H), 7.34(m ,2H),4.08(m,2H),3.72(s,3H),2.42(m,1H),1.16(m,2H),0.85(m,2H)
[0085]...
Embodiment 2
[0094]
[0095] (1S,2R)-2-Amino-1,2-diphenylethanol
[0096] Mix 10g of racemic lesinurad (1.0eq), 5g of chiral aminoalcohol derivative (1S,2R)-2-amino-1,2-diphenylethanol (1.0eq), and 65mL of acetone, and heat in an oil bath to 60°C, dissolve until clear. React at 60°C for 1h, stop heating, slowly cool to 7-8°C in an oil bath, filter, and wash the filter cake with ethyl acetate (EA) to obtain 4.05g of white solid powder A (salt of lesinurad in R configuration) , ee=98.6%. The filtrate was concentrated by rotary evaporation and dried to obtain 10.9 g of white solid C, ee=24.4% (the crude salt of S configuration lesinurad).
[0097] Add 4.05g of the white solid powder A in the above step to 230mL of ethyl acetate / acetone=10 / 1 mixed solvent, set the oil bath at 70°C, heat and dissolve until clear, stop heating after 1h, and slowly cool in the oil bath To 7-8°C, filter, and wash the filter cake with ethyl acetate to obtain 3.57 g of white solid powder B (salt of lesinurad i...
Embodiment 3
[0100]
[0101] (1S,2R)-2-Amino-1,2-diphenylethanol
[0102] Referring to Example 2, 5g racemic lesinurad (1.0eq), 3.9g chiral aminoalcohol derivative (1S,2R)-2-amino-1,2-diphenylethanol (1.5eq), 250mL ethyl acetate / acetone=10 / 1 mixed, heated to 80°C in an oil bath, dissolved until clear. React at 80° C. for 1 h, stop heating, slowly cool to 28° C. in an oil bath, filter, and wash the filter cake with ethyl acetate to obtain 2.2 g of white solid powder (salt of lesinurad in R configuration), ee=96.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com