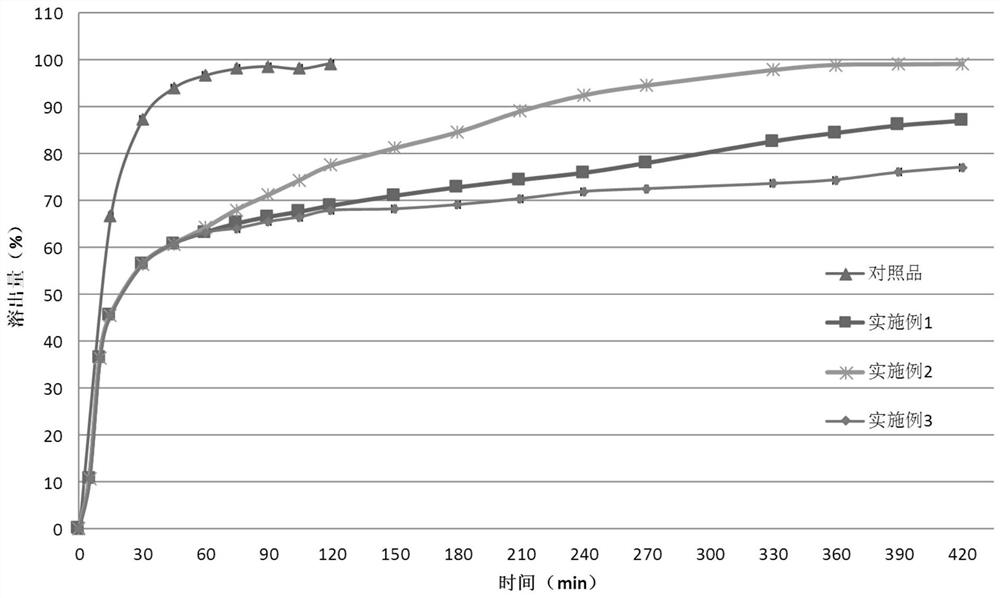
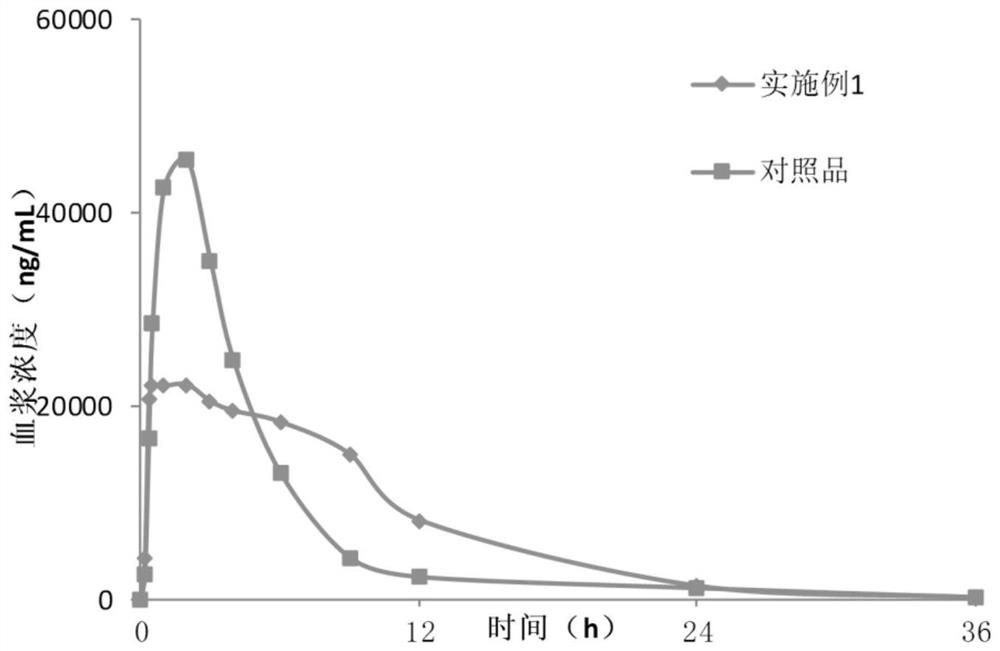
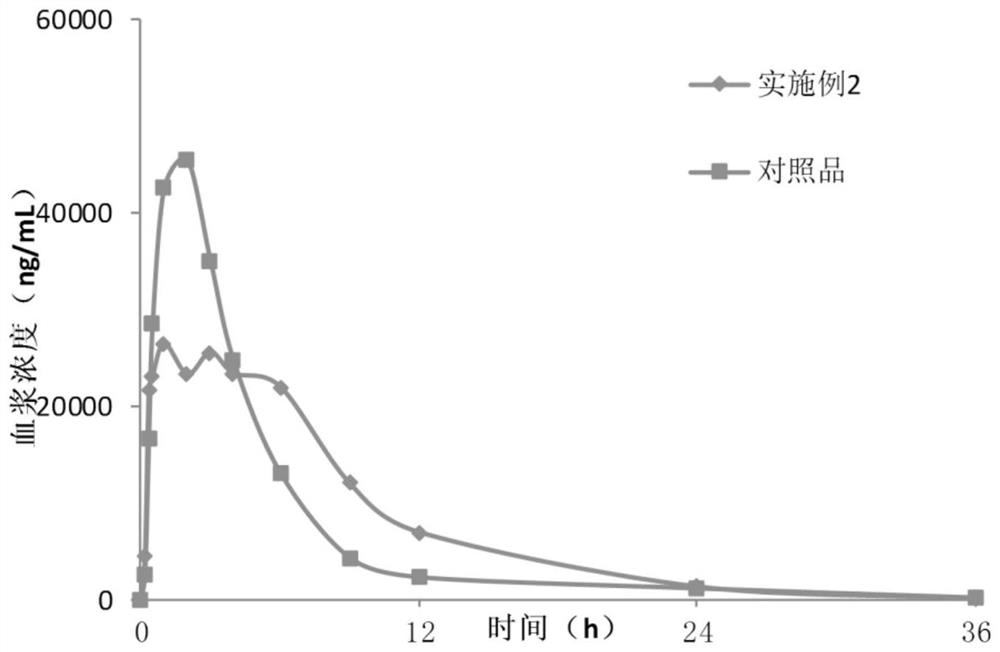
Lesinurad controlled-release pharmaceutical composition
A technology for controlled release of drugs and compositions, applied in the directions of drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problems of harsh drug taking conditions, toxic and side effects of Resinad, and reduce the risk of drug use, The effect of enhancing drug efficacy, improving release and dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Table 1 Sustained-release component formula (100 tablets)
[0050]
[0051]
[0052] Table 2 Immediate Release Component Formulation (100 Tablets)
[0053] Raw materials Content per tablet (mg) Proportion(%) Prescription dosage (g) Recinard 150.00 66.67 15.000 HPMC E5 4.50 2.00 0.450 lactose 33.25 14.78 3.325 MCC102 23.75 10.56 2.375 Crospovidone 11.25 5.00 1.125 Magnesium Stearate (additional) 2.25 1.00 0.225 total 225.00 100.00 22.500
[0054] (1) prepare sustained-release component according to the formula of table 1:
[0055] 1) Mixing: Mix the weighed Recinard, HPMC E50LV, lactose, and MCC102 for 3 minutes, pass through a 24-mesh sieve, and mix for another 3 minutes;
[0056] 2) Granulation: adding a wetting agent (50% ethanol), wet granulation, and passing through a 24-mesh sieve;
[0057] 3) Drying: drying in an oven at 60°C, moisture <3%;
[0058] 4) Grain sizing: dry granules p...
Embodiment 2
[0068] Table 3 Sustained Release Component Formula (100 Tablets)
[0069] Raw materials Content per tablet (mg) Proportion(%) Prescription dosage (g) Recinard 100.00 36.36 10.000 HPMC E50LV 55.00 20.00 5.500 lactose 78.18 28.43 7.818 MCC102 39.07 14.21 3.907 Magnesium Stearate (additional) 2.75 1.00 0.275 total 275.00 100.00 27.500
[0070] Table 4 Immediate Release Component Formulation (100 Tablets)
[0071] Raw materials Content per tablet (mg) Proportion(%) Prescription dosage (g) Recinard 150.00 66.67 15.000 HPMC E5 4.50 2.00 0.450 lactose 33.25 14.78 3.325 MCC102 23.75 10.56 2.375 Crospovidone 11.25 5.00 1.125 Magnesium Stearate (additional) 2.25 1.00 0.225 total 225.00 100.00 22.500
[0072] According to the method in Example 1, the sustained-release component and the immediate-release component were prepared, and then double-la...
Embodiment 3
[0074] Table 5 Immediate Release Component Formulation (1000 capsules)
[0075] Raw materials Content per tablet (mg) Proportion(%) Prescription dosage (g) Recinard 150.00 66.67 150.00 Ball core 56.66 28.33 56.66 Hypromellose 10.00 5.00 10.00 total 200.00 100.00 200.00
[0076] Table 6 Sustained-release component formula (1000 grains)
[0077] Raw materials Content per tablet (mg) Proportion(%) Prescription dosage (g) Recinard 100.00 36.36 100.000 Ball core 110.00 33.64 110.00 Hypromellose 15.00 5.00 15.00 Surelease 75.00 25.00 75.00 total 300.00 100 300.00
[0078] (1) According to the formula preparation of table 5 release component immediately:
[0079] 1) Preparation of the medicinal solution: add the adhesive to an appropriate amount of water, stir until it is completely dissolved, add Recinard, continue to stir, and suspend evenly.
[0080] 2) Preparation of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com