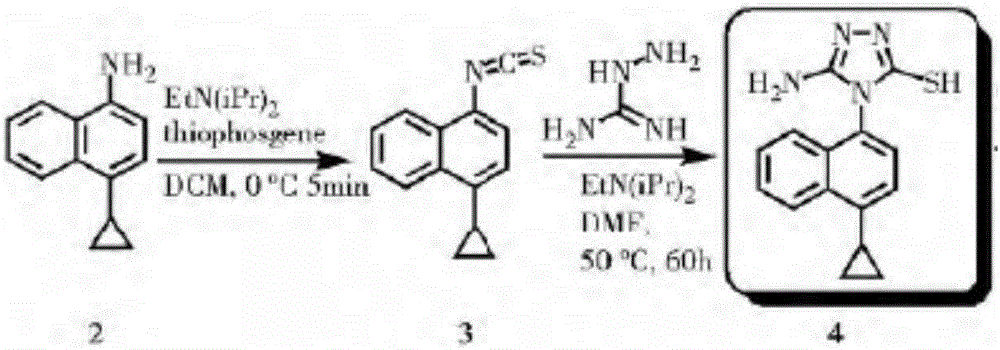
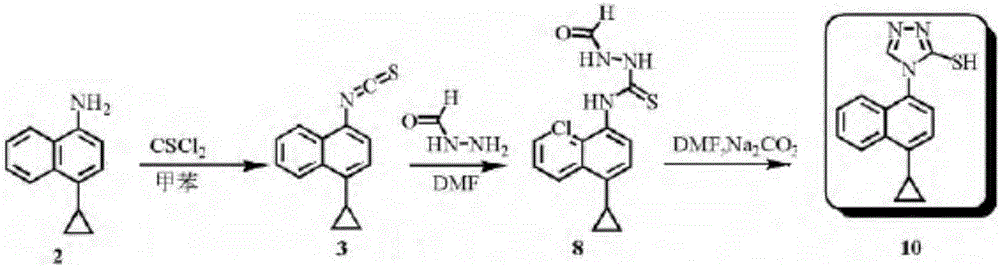
Method for preparing lesinurad intermediate
A technology for lesanolide and intermediates, which is applied in the field of preparation of lesinolide intermediates, can solve the problems of many reaction steps, achieve simple preparation, avoid the use of toxic reagents such as thiophosgene, and have low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1 Synthetic route of lesanolide of the present invention
[0063]
[0064] step one:
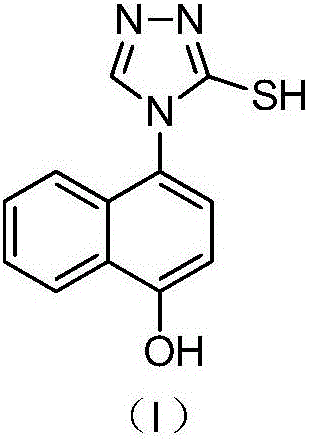
[0065]In a 100ml three-necked flask equipped with a stirring bar and a thermometer, add 50ml of ethanol and 2.23g of 4-bromonaphthol, stir at room temperature, add 1.01g of 3-mercapto-1,2,4-triazole until it dissolves with stirring, and control Add 0.4g of sodium hydroxide at 30°C, keep stirring for 3 hours, concentrate to remove ethanol, add 25ml of purified water and 25ml of dichloromethane for extraction and separation, concentrate the organic layer to remove dichloromethane, add 25ml of acetone to the residue, stir and crystallize to obtain 2.34g product, ie intermediate (I), yield 96.30%.
[0066] Step two:
[0067] In a 100ml three-necked flask equipped with a stirrer and a thermometer, add 50ml of dichloromethane, 2.43g of intermediate (I), drop 3 drops of DMF, and slowly add 1.8g of thionyl chloride; after the addition, heat up to reflux and maintain reflux After...
Embodiment 2
[0076] Embodiment 2 Synthetic method of lesinolide of the present invention
[0077] step one:
[0078] In a 100ml three-neck flask equipped with a stirring bar and a thermometer, add 50ml of ethanol and 2.23g of 4-bromonaphthol, stir at room temperature, add 1.01g of 3-mercapto-1,2,4-triazole until it dissolves with stirring, and control Add 0.4g of triethylamine at 30°C, keep stirring for 3 hours, concentrate to remove ethanol, add 25ml of purified water and 25ml of dichloromethane for extraction and separation, concentrate the organic layer to remove dichloromethane, add 25ml of acetone to the residue, stir and crystallize to obtain 2.30g product, ie intermediate (I), yield 94.65%.
[0079] Step two:
[0080] In a 100ml three-necked flask equipped with a stirrer and a thermometer, add 50ml of dichloromethane, 2.43g of intermediate (I), drop 3 drops of DMF, and slowly add 1.8g of thionyl chloride; after the addition, heat up to reflux and maintain reflux After 4 hours, it...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com