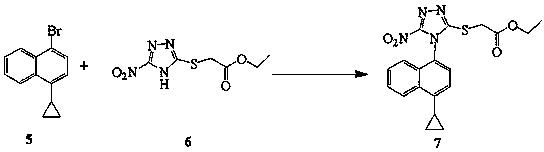
Preparing method for (4-(4-cyclopropyl-naphthalene-1-yl)-5-nitr-4H-(1, 2, 4) triazole-3-ylsulfanyl)-ethyl acetate and intermediate (5-nitr-4H-(1, 2, 4) triazole-3-sulfenyl)-ethyl acetate thereof
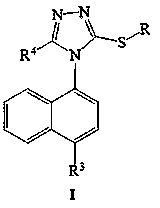
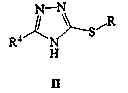
An intermediate, cyclopropyl technology, applied in [4-(4-cyclopropylnaphthalen-1-yl)-5-nitro-4H-[1,2,4]triazol-3-ylthio The preparation of alkyl]-ethyl acetate and its intermediate (5-nitro-4H-[1,2,4]triazol-3-ylsulfanyl)-ethyl acetate field can solve the problem of long synthetic route and environmental problems. Large impact, inconvenient operation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Preparation of (4H-[1,2,4]triazol-3-ylthio)-ethyl acetate
[0027] Weigh (10.5 g; 103 mmol) 4H-[1,2,4]triazole-3-thiol, (15.7 g; 129 mmol) ethyl chloroacetate into a 500 mL three-necked flask, add 250 mL ethyl acetate , (31.5 g; 3 eq) triethylamine, heated up to 50 °C, LC followed the reaction, the raw material was completely converted in about 1.5 h, filtered with suction, and the insoluble matter in the reaction solution was removed, and the mother liquor was concentrated under reduced pressure at 50 °C to obtain light Yellow oily concentrate 18.1 g, yield 95%.
Embodiment 2
[0028] Example 2: Preparation of (5-nitro-4H-[1,2,4]triazol-3-ylthio)-ethyl acetate
[0029] Add (55 mL; 5 vol) acetic anhydride to the above concentrate, cool down to 0°C, and slowly add 40 mL of 20% sodium nitrite solution dropwise for 8 h, then rise to room temperature and stir for two hours after the drop is completed, and the reaction solution is Reduce to 0°C, add 100 mL of purified water dropwise, a yellow solid precipitates, stir at 0°C for 1 h, filter with suction, wash the filtrate with purified water until neutral, and dry under vacuum at 80°C to obtain 16.5 g of a yellow solid, yield 75% .
Embodiment 3
[0030] Example 3: Preparation of [4-(4-cyclopropylnaphthalen-1-yl)-5-nitro-4H-[1,2,4]triazol-3-ylsulfanyl]-ethyl acetate
[0031] Weigh (12.5 g; 54 mmol) of the above yellow solid, (15.9 g; 64.8 mmol) 4-cyclopropyl-1-bromonaphthalene, (3.4 g; 20% mmol) CuI 2 , (22.9 g; 2 eq) K 3 PO 4 , (195 mg, 20% mmol) H 2 O, (1.6 g; 20%mmol) 8-hydroxyquinoline, 100 mL of toluene was added to a 250 mL three-necked flask, nitrogen protection, the temperature was raised to reflux, LC followed the reaction, the raw material was less than 3% for about 16 h, the reaction was processed, and the The reaction solution was lowered to 10 °C, under the protection of nitrogen, 100 mL of purified water, 60 mL of methyl tert-butyl ether, and 125 mL of n-heptane were added to the reaction solution, and the temperature was kept below 10 °C, stirred for two hours, and suction filtered , the filter cake was washed twice with 30 mL of methyl tert-butyl ether, and vacuum-dried at 80 °C to obtain 13.9 g of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com