Anti-morphine monoclonal antibody, cell strain for generating anti-morphine monoclonal antibody, morphine detection kit and manufacturing method thereof
A monoclonal antibody and kit technology, applied in the biological field, can solve the problems of inconvenient sampling, late window period, dirty samples, etc., and achieve the effect of detection limit advantage, strong affinity and high specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: Preparation of morphine artificial antigen
[0042]
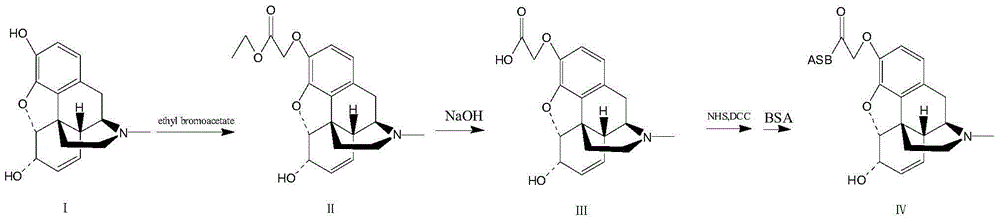
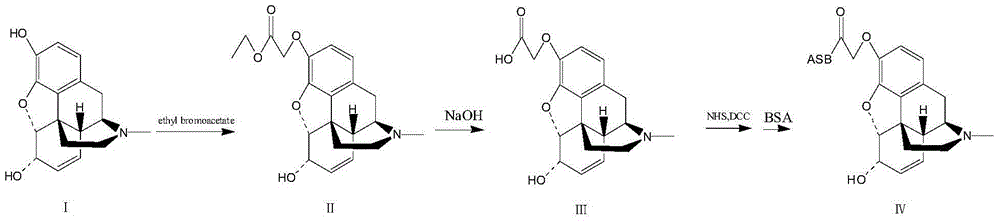
[0043] The above formula is the synthetic route of morphine artificial antigen.
[0044] 1-1. Preparation of morphine hapten: use morphine hydrochloride as raw material, carry out nucleophilic substitution reaction with ethyl bromoacetate after dissociation, and obtain carboxyl-containing morphine hapten.
[0045] Dissolve 350mg of morphine hydrochloride in 10ml of water, adjust the pH to 10 with concentrated ammonia water, a large amount of solids precipitate, place it at 4°C for 30 minutes, and centrifuge at 10,000 rpm to remove the clear liquid The water and ethanol were transferred to a round bottom flask, and concentrated under reduced pressure at 50°C to obtain 288 mg of white solid morphine.
[0046] In a 100ml three-necked flask, 285mg (1mmol) of morphine and 10ml of DMF were added, and under nitrogen protection, 330mg (2.4mmol) of potassium carbonate and 184mg (1.1mmol) of ethyl 4-bromoacet...
Embodiment 2
[0050] Example 2: Screening of anti-morphine monoclonal antibody hybridoma cell lines
[0051] Step (1) mouse immunization
[0052] Morphine artificial antigen, Freund's adjuvant, and 3% Tween-80 saline were mixed according to the volume of 1:1:1 to obtain emulsion by stirring method. Select 8-week-old BALB / c female mice for subcutaneous immunization combined with intrasplenic and intravenous immunization for a total of 5 immunizations. The immunization time is the 1st day, the 14th day, the 21st day, the 22nd day, and the 23rd day. The specific steps Eight-week-old BALB / c female mice were immunized for the first time, and 50 μg of morphine artificial antigen emulsion (complete Freund's adjuvant was used as the adjuvant) was injected into the subcutaneous part near the lymph nodes; for the second intrasplenic immunization, Morphine artificial antigen emulsion with an immune dose of 20 μg (incomplete Freund’s adjuvant is used as an adjuvant); the third, fourth, and fifth times...
Embodiment 3
[0078] Example 3: Performance testing of anti-morphine monoclonal antibody
[0079]3.1 Anti-morphine monoclonal antibody subtype identification
[0080] The subtype of the anti-morphine monoclonal antibody prepared in Example 1 was identified with reference to the monoclonal antibody typing kit. The anti-morphine monoclonal antibody belonged to IgG1 subtype, and the light chain was kappa chain.
[0081] 3.2 Determination of affinity constant of anti-morphine monoclonal antibody
[0082] Example of Affinity Determination Using Antibody Competitive Binding to Antigen
[0083] ① Dilute the morphine monoclonal antibody (MOP-BSA) to 1 μg / ml with carbonate buffer solution with a pH of 9.6 and a concentration of 0.05M, and then add 100 μl of diluted morphine artificial antigen to each well of a 96-well microtiter plate, Coat overnight at 4°C (2 plates); then wash the plate 3 times with PBS buffer containing 0.05% Tween-20 and pat dry; ② Add 200 μl 0.01mol / L pH7.2 phosphate containi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Affinity constant | aaaaa | aaaaa |
| Affinity constant | aaaaa | aaaaa |
| Affinity constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com