Synthesis method of tirbanibulin
A synthetic method, Teban's technology, applied in the synthesis field of terbanbulin, can solve the problems of long route, increased production cost of terbanbulin, cost of raw materials, high cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
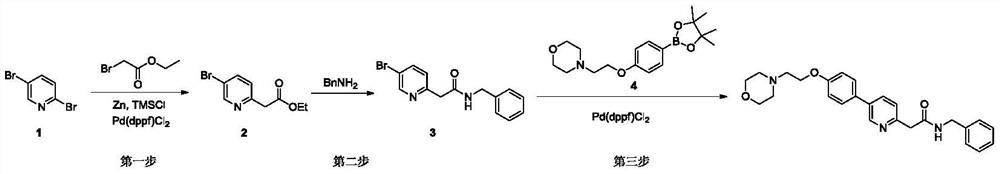
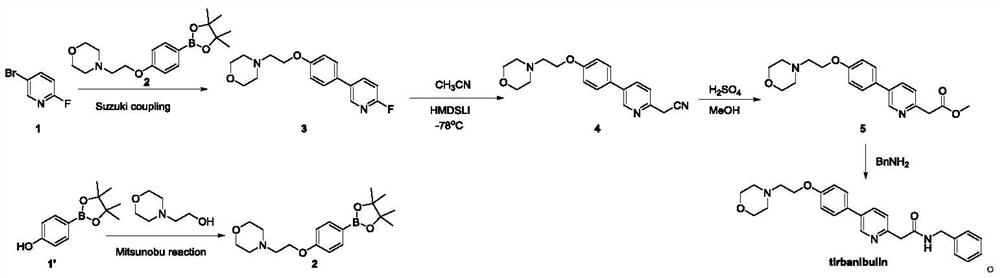
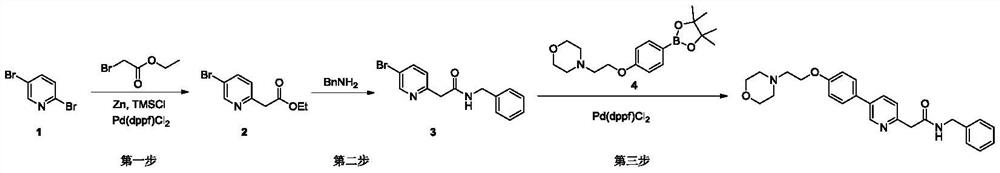
[0025] The present embodiment provides a kind of method of synthesizing terbanbuline, and the reaction equation is:
[0026]
[0027] The synthesis process is as follows:
[0028] The first step: at room temperature (about 25°C), add 253.8g of ethyl bromoacetate to 3L of anhydrous 1,4-dioxane solution, then cool down to 0°C, add 10g of newly activated zinc powder , then add 0.2g of trimethylchlorosilane (TMSCl) under stirring, stir for 30 minutes, then add 88.8g of zinc powder in batches, slowly heat up under stirring, and control the heating rate at about 1°C per minute until heated to 100 ℃, the zinc powder disappeared completely; the reaction system was cooled to 0 ℃, 300g of 2,5-dibromopyridine and 11.2g of 1,1'-bisdiphenylphosphinoferrocene palladium (PdCl2) were added in batches (dppf)Cl 2 ), then slowly warmed to room temperature, reacted for 3 hours, and then slowly warmed to 100 °C and reacted overnight. Cool the reaction solution to 0°C, then add 500 ml of ammo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com