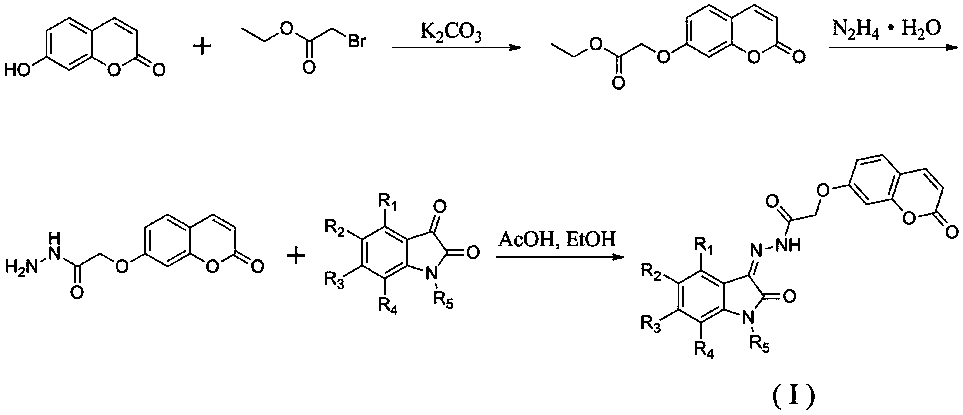
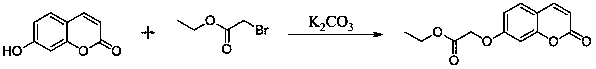Coumarin-isatin type compounds, and preparation method and purpose thereof
A compound, coumarin technology, applied in organic chemistry, drug combination, metabolic diseases, etc., can solve the problems of high toxicity and side effects, low activity, hindering application, etc., and achieve a good effect of inhibiting activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Preparation of ethyl 2-((2-oxo-2H-benzopyran-7-yl)oxy)acetate
[0021]
[0022] Put 7-hydroxycoumarin (1.62g, 10mmol), ethyl bromoacetate (1.84g, 11mmol), potassium carbonate (4.15g, 30mmol) in a round bottom flask, add acetone 100mL, reflux for 6 hours, TLC It shows that the reaction is complete, stop the reaction, cool to room temperature, remove potassium carbonate by filtration, spin the filtrate to dryness, and recrystallize with ethanol to obtain a white solid powder with a yield of 86.4%. m.p.107-110 o c.
Embodiment 2
[0023] Example 2: Preparation of 2-((2-oxo-2H-benzopyran-7-yl)oxy)acetohydrazide
[0024]
[0025] Place ethyl 2-((2-oxo-2H-benzopyran-7-yl)oxy)acetate (2.48g, 10mmol), hydrazine hydrate (1.0g, 20mmol) in a round bottom flask , add 100mL ethanol, reflux reaction, TLC shows that the reaction is complete, stop the reaction, cool to room temperature, filter to obtain a white solid powder, the yield is 65.3%. m.p.171-175 o c.
Embodiment 3
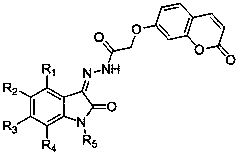
[0026] Example 3: (Z)-2-((2-oxo-2H-benzopyran-7-yl)oxy)-N'-(2-oxoindolane-3-ylidene)acetyl Preparation of hydrazine (1)
[0027]
[0028] Put 2-((2-oxo-2H-benzopyran-7-yl)oxy)acetohydrazide (234mg, 1mmol), isatin (147mg, 1mmol) in a round bottom flask, add 10mL of methanol and A drop of glacial acetic acid was refluxed for 2 hours to stop the reaction, cooled to room temperature, and filtered to obtain a yellow solid powder with a yield of 87.4%. m.p.237-243 o C; 1 HNMR(d 6 -DMSO,400MHz)δ:5.01(s,2H),6.32(d,1H,J=9.6Hz),6.94(d,1H,J=8.0Hz),7.05-7.10(m,3H),7.37(d ,1H,J=8.4Hz),7.58-7.68(m,2H),8.00(d,1H,J=9.6Hz),10.23(s,1H),11.32(s,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com